Percutaneous transluminal angioplasty and stent placement (PTAS) has become a treatment option for selected patients with symptomatic intracranial arterial stenosis.1 The Stent Placement versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial was initiated in October of 20082 to compare PTAS with aggressive medical managements. The intent of the PTAS treatment was to prevent the primary end point (any stroke or death within 30 days after enrollment or stroke in the territory of the symptomatic intracranial artery beyond 30 days) during a mean follow-up of 2 years in high-risk patients with symptomatic stenosis of a major intracranial artery. Details about the study design, patient enrollment, and follow-up have been published previously.2 The study was powered to detect a relative 35% reduction in the rate of the primary end point from stent placement based on the log-rank test with an α of .05% and 80% power.
On April 5, 2011, the trial was prematurely halted at the recommendation of the Safety Monitoring Board appointed by the National Institutes of Health. This decision was based on the considerably high 30-day stroke and death rate in the PTAS group (14%) compared with the medical management group (5.8%).3 The 30-day rate of stroke or death in the PTAS group was substantially higher than the rates previously reported (rates ranging from 4.4% to 9.6%).4,5 Of the 33 strokes in the PTAS group that occurred within 30 days after enrollment, 25 occurred within 1 day after the procedure and 8 occurred 2–6 days later.3 These results raised the question of whether the rate of 30-day events can be reduced in patients undergoing PTAS by more appropriate patient selection and greater operator experience. Another important question is: What is the rate of 30-day events required for PTAS to be beneficial in reducing the long-term rate of ipsilateral stroke?
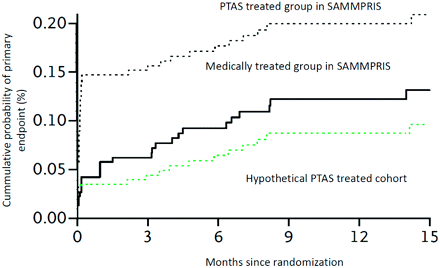
We performed an analysis on the basis of the results provided by SAMMPRIS to determine the 30-day rate of stroke and/or death required to achieve a 35% relative risk reduction of the primary end point (composite of 1 month stroke and/or death and ipsilateral stroke beyond 1 month) among the PTAS-treated group compared with the medically treated group at 1 year follow-up. Our analysis found that if the 30-day rate of stroke and/or death had been 3.8% in the PTAS group, then the relative risk reduction by PTAS would have met the primary hypothesis of the trial (Fig 1). The analysis assumes that the rate of events in patients treated with PTAS after 1 month and the rate in those treated medically are the same as that observed in SAMMPRIS. Less than half of the patients had been followed for 1 year, which may result in underestimation of ischemic events in patients who are medically managed. Our analysis supports the notion that if perioperative stroke and/or death in patients treated with PTAS can be reduced to <4%, PTAS has the potential to reduce the rate of recurrent ipsilateral stroke in patients with ipsilateral ischemic events and high-grade stenosis (70%–99%).
Kaplan-Meier curves for the cumulative probability of the primary end point after randomization in the PTAS and the medically treated groups in the SAMMPRIS trial and the probability in a hypothetical PTAS-treated cohort that provides the 35% relative risk reduction with PTAS treatment compared with medical treatment.
Alternatively, the medical community may consider a lower relative risk reduction with PTAS as clinically meaningful. The question is whether such periprocedural stroke and/or death rates can be achieved in PTAS procedures performed in selected settings and with careful selection of patients based on lesion characteristics and anatomic locations. Another issue that needs to be considered is the mandatory policy of stent placement once the patients were randomized into the PTAS group despite the anatomic factors such as proximal tortuosity and vessel dimensions. Primary angioplasty alone has been used for treatment of symptomatic intracranial stenosis and appears to be associated with lower periprocedural stroke and death rates6 and should be considered as an alternative to stent placement. The threshold of a 4% rate of periprocedural stroke and/or death with PTAS that is required to provide clinical benefit is probably the new standard for current clinical practice and future clinical trials.
Footnotes
-
Disclosures: Adnan Qureshi has received funding from the National Institutes of Health RO-1-NS44976–01A2 (medication provided by ESP Pharma, Edison, NJ) and the American Heart Association Established Investigator Award 0840053N, National Institutes of Health UO1-NS062091-O1A2, and the Minnesota Medical Foundation, Minneapolis, Minnesota.
References
- © 2011 by American Journal of Neuroradiology