Abstract
BACKGROUND AND PURPOSE: Emerging evidence suggests that obese adolescents show changes in brain structure compared with lean adolescents. In addition, obesity impacts body development during adolescence. We tested a hypothesis that T1, a marker of brain maturation, can show brain differences associated with obesity.
MATERIALS AND METHODS: Adolescents similar in sex, family income, and school grade were recruited by using strict entry criteria. We measured brain T1 in 48 obese and 31 lean adolescents by quantitative MR imaging at 1.5T. We combined MPRAGE and inversion-recovery sequences with normalization to standard space and automated skull stripping to obtain T1 maps with a symmetric voxel volume of 1 mm3.
RESULTS: Sex, income, triglycerides, total cholesterol, and fasting glucose did not differ between groups, but obese adolescents had significantly lower HDL, higher LDL, and higher fasting insulin levels than lean adolescents. Intracranial vault volume did not differ between groups, but obese adolescents had smaller intracranial vault-adjusted brain parenchymal volumes. Obese adolescents had 4 clusters (>100 contiguous voxels) of T1 relaxation that were significantly different (P < .005) from those in lean adolescents. Three of these clusters had longer T1s in obese adolescents (in the orbitofrontal and parietal regions), and 1 cluster had shorter T1s, compared with lean adolescents.
CONCLUSIONS: Our results suggest that obesity may have a significant impact on brain development, especially in the frontal and parietal lobes. It is unclear if these changes persist into adulthood or whether they indicate that obese subjects follow a different developmental trajectory during adolescence.
ABBREVIATIONS
- BMI
- body mass index
- FDR
- false discovery rate
- GM
- gray matter
- HDL
- high density lipoprotein
- HOMA-IR
- homeostatic model assessment index of insulin resistance
- LDL
- low density lipoprotein
- MAP
- mean average blood pressure
- MPRAGE
- magnetization-prepared rapid acquisition of gradient echo
- OFC
- orbitofrontal cortex
- SES
- socioeconomic status
- T1
- spin-lattice relaxation time
- VANCOVA
- voxelwise analysis of covariance
MR imaging provides unique opportunities for in vivo study of the living human brain. For more than a decade, animal and human measurements of T1, a physical parameter of protons, have contributed significantly to the investigation of brain maturation, with T1 shortening occurring during the process of maturation.1⇓⇓–4 In general, T1 increases with increasing tissue water content and decreases in tissues high in lipid content. Water and lipid content, therefore, provide inherent MR imaging contrast agents to distinguish tissue types.
In normal-appearing WM, long T1 can reflect tissue damage related to brain edema or a loss in the integrity of axonal myelin sheaths. For example, T1 can be used as a marker of prognosis in primary-progressive multiple sclerosis,5 because T1 of the tissue increases in association with an increase in water content from inflammation or a decrease in lipid content from axonal loss. Steen et al1 demonstrated that T1 can also be used as a measure of normal brain maturation, and unlike other MR imaging quantitative techniques used to assess changes in the developing human brain, T1 remains sensitive to changes throughout adolescence and into adulthood. Steen et al6 used radio-frequency saturation of inflowing blood spins to show that perfusion cannot account for T1 abnormalities in GM, even when blood flow is rapid or the vascular volume is as high as 5%. This suggests that brain T1 measurements reflect brain parenchyma, not blood artifacts.
To the best of our knowledge, there are no previous studies that assess brain maturation, characterized by T1 relaxation, in obese adolescents relative to lean adolescents. This is of interest because we know that obesity leads to faster sexual and bone maturation in boys and girls,7,8 but little is known about the impact of obesity on brain development and maturation. Whether obesity affects brain maturation is an important question, given that there is emerging evidence that obese adolescents show regional volume changes, such as smaller OFC volumes associated with higher ratings of disinhibition in eating behaviors9 relative to well-matched lean adolescents. In addition, obesity-induced type 2 diabetes is associated with significant reduction in microstructural integrity assessed by DTI10 as well as hippocampal and frontal lobe volume reductions.11 These observations suggest that obesity may impact brain development during adolescence and that obese adolescents may follow a different neurodevelopmental trajectory relative to lean adolescents. To explore this hypothesis, we used T1 as a marker of brain maturation and integrity.
We hypothesized that obese adolescents, given their accelerated sexual development, will have a parallel acceleration of brain maturation and, thus, a reduction of T1 in GM and WM tissue relative to lean adolescents. However, we were also interested in ascertaining whether the metabolic abnormalities that are associated with regional brain volume reductions and loss of DTI-based microstructural integrity9 could affect measures of brain maturation.
Materials and Methods
Participants
The protocol was approved by the Institutional Research Board of the New York University School of Medicine. All parents of adolescents younger than 18 years of age provided written informed consent, and children assented to the study and were compensated for their time and inconvenience.
Seventy-nine adolescent volunteers (31 lean and 48 obese) were consecutively studied. Participants were recruited via Internet advertisements or were friends or relatives of other study participants. We used standardized criteria for study entry to have groups representing quite a narrow age range (from 14 to 21 years) and groups comparable in sex, school grade, ethnicity, and SES (personal communication, A.B. Hollingshead, Department of Sociology, Yale University, New Haven, Connecticut, 1975). Subjects were screened to rule out medical problems (other than hypertension, dyslipidemia, polycystic ovary disease, or insulin resistance) and any possible neurologic/psychiatric confound (eg, history of head trauma, use of psychoactive medication, significant drug use, significant learning disability, or depression). Only adolescents with sexual development Tanner stage 4 or greater, equivalent to an average age of about 16, were included in the study. None of the participants had dental braces. A comprehensive panel of blood tests was performed after a 10- to 12-hour overnight fast for the assessment of blood count, liver and lipid profile, thyroid function, blood chemistries, glucose levels, insulin levels, and high-sensitivity C-reactive protein levels. Fasting glucose and insulin values were used to compute HOMA-IR, which has been shown to be a valid surrogate of insulin sensitivity,12 with higher HOMA-IR values indicating more insulin resistance. BMI, computed as the weight in kilograms divided by height in meters squared, was used to characterize lean and obese individuals. Adolescents were considered lean if they had a BMI between 18 and 25 kg/m2; those with a BMI of ≥30 kg/m2 were in the obese category. Adolescents (n = 2) who had a BMI between 25.1 kg/m2 and 29.9 kg/m2 were considered overweight and were not included in the study. Waist circumference was also measured to ensure that extremely muscular adolescents, who could have a high BMI but are not likely to have abdominal obesity, were appropriately classified. All of our adolescents who met the BMI criteria for obesity also had abdominal obesity as indicated by a waist circumference >88 cm for girls and >102 cm for boys. We computed the MAP, which is a weighted average of the systolic and diastolic blood pressures [MAP = (2/3 × diastolic + 1/3 × systolic)], as a measure of average arterial pressure for each participant.
MR Imaging
Image Acquisition.
MR imaging was performed using a 1.5T Avanto (Siemens, Erlangen, Germany) MR imaging system. A structural T1-weighted MPRAGE sequence (TR = 1300 ms, TE = 4.38 ms, TI = 800 ms, NEX = 1, FOV = 250 × 250, 196 coronal sections, section thickness = 1.2 mm, flip angle = 15°) was acquired. A set of turbo spin-echo inversion-recovery sequences (TR = 10 000 ms; TE = 9.7 ms; TI = 23, 305, 675, 1100, and 2000 ms; FOV = 210 × 210; 50 axial sections; section thickness = 3 mm; 1 average refocusing pulse; flip angle = 145°), with an acquisition time of 3 minutes each, was collected at identical section positions for T1 measurement. The sections were collected as 2 concatenations, (ie, odd sections were sampled during an initial 10-second TR period; even sections were sampled during a second 10-second TR period). The axial plane of acquisition was set parallel to a plane that goes through the inferior aspect of the orbitofrontal cortex and the bottom of the occipital cortex on the midsagittal plane. A T2-weighted sequence (TR = 9000 ms; TE = 94 ms; FOV = 210 × 210 mm; 50 sections; section thickness = 3 mm) was also acquired in the same orientation, with the same section thickness and number of sections to maximize section coregistration. FLAIR images (TR = 9000 ms; TE = 97 ms; FOV = 210 × 210 mm; 50 sections; section thickness = 3 mm) were collected to quantify WM disease by using the modified Fazekas scale13 to rate WM hyperintensities.
T1 Mapping.
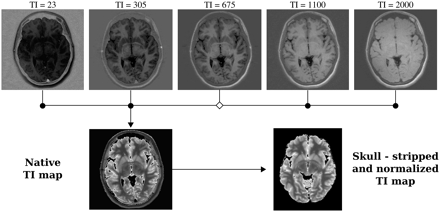
Images were processed by using the in-house Multimodal Imaging Data Analysis System and Automated Registration Toolkit 2.10 Individual structural native space images were first manually skull-stripped and normalized to standard space to fit a 3D warp field containing the MPRAGE images to target transformation parameters.14 Next, we applied a rigid-body registration of the T2-weighted volume to the MPRAGE volume to generate the T2-weighted-to-MPRAGE transformation matrix.15 We then combined the transformation parameters and the transformation matrix to skull strip and spatially normalize the T1 maps. As a result, T1 maps ended up with a symmetric voxel size of 1 × 1 × 1 mm3.
The T1 maps were generated from the native inversion-recovery scans with varying TIs (23, 305, 675, 1100, and 2000 ms). These inversion-recovery image sets were coregistered to the set acquired with a TI = 675, namely the one with the best GM/WM contrast. T1 was calculated on a pixel-by-pixel basis by using programs written in-house in IDL (ITT Visual Information Solutions, Boulder, Colorado) with standard algorithms. In phased inversion-recovery, image signal intensity S is given by
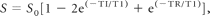 where S0 is the unsaturated signal intensity, TI is the inversion time, and TR is the repetition time. Pixel signal-intensity values were fitted to the equation by using a gradient expansion method with T1 and S0 as fitting parameters. Given the very long TR used in this acquisition, the final term in the equation is not strictly necessary. The inversion pulse was adiabatic, which, coupled with the cosine dependence of inversion, gives a very nearly ideal inversion efficiency (ie, the factor 2 in the second term of the equation is accurate). Inaccuracy of the initial excitation pulse leads only to a change in S0 that is equal for all inversion times and, therefore, causes no error in T1 estimation. For the same reason, it is unnecessary to take into account nonideal refocusing pulses. Furthermore, the effect of refocusing on longitudinal magnetization can be neglected because TR is very much greater than TE. Finally, any residual error in T1 estimation will apply equally to both subject and control groups, so error cannot affect the overall conclusions. A noise threshold and T1 thresholds of 1500 (maximum) and 100 ms (minimum) were applied to exclude pixels that were either background, CSF, or interfaces such as air/bone or brain/CSF. The use of a high-resolution turbo spin-echo in the inversion- recovery sequences minimizes the effects of magnetic susceptibility.
where S0 is the unsaturated signal intensity, TI is the inversion time, and TR is the repetition time. Pixel signal-intensity values were fitted to the equation by using a gradient expansion method with T1 and S0 as fitting parameters. Given the very long TR used in this acquisition, the final term in the equation is not strictly necessary. The inversion pulse was adiabatic, which, coupled with the cosine dependence of inversion, gives a very nearly ideal inversion efficiency (ie, the factor 2 in the second term of the equation is accurate). Inaccuracy of the initial excitation pulse leads only to a change in S0 that is equal for all inversion times and, therefore, causes no error in T1 estimation. For the same reason, it is unnecessary to take into account nonideal refocusing pulses. Furthermore, the effect of refocusing on longitudinal magnetization can be neglected because TR is very much greater than TE. Finally, any residual error in T1 estimation will apply equally to both subject and control groups, so error cannot affect the overall conclusions. A noise threshold and T1 thresholds of 1500 (maximum) and 100 ms (minimum) were applied to exclude pixels that were either background, CSF, or interfaces such as air/bone or brain/CSF. The use of a high-resolution turbo spin-echo in the inversion- recovery sequences minimizes the effects of magnetic susceptibility.
Volumetric Data.
The MPRAGE scans were used to determine the intracranial vault size, which was obtained by manually outlining the supratentorial compartment. This was done by outlining the margins of the dura and the tentorium on the saggital images as described in detail elsewhere.16 We computed total brain parenchyma volumes after setting an individualized threshold to exclude for the CSF component. We residualized brain parenchyma volume to the intracranial vault at the case level to control for sex and individual variability in head size from body stature.
Statistical Analyses
We performed 2-tailed independent-samples t tests for demographic and endocrine data. We used the χ2 test to examine the group differences in sex and SES.
To test for possible changes in brain T1 among obese adolescents relative to lean adolescents, we conducted a 2-tailed VANCOVA. To reduce the risk of escalation of type 1 error due to multiple comparisons of the imaging data, only clusters of at least 100 contiguous significant voxels (0.1 mL) were identified as significantly different between groups. We chose an FDR <1%. We selected an appropriate significance threshold that ensured that the FDR was <0.01. We controlled for age to minimize the slight age difference between groups. To account for group differences in brain volumes leading to segmentation errors, we also controlled for intracranial vault-adjusted brain parenchymal volume. In addition, we controlled for the MAP, because blood pressure is associated with lacunae and hyperintensities and might affect T1 contrast. The clusters were registered to the standard Montreal Neurologic Institute T1 MR imaging template and visualized with Analysis of Functional Neuroimages (http://afni.nimh.nih.gov/). The clusters resulting from the voxelwise analyses were mapped back to the individual case T1 maps (in native space) to then compute the average T1 for each cluster.
Results
Table 1 summarizes the demographic and endocrine data. None of the participants had any hyperintensities or lacunae on the FLAIR images. The groups were matched in sex, but obese adolescents were slightly older than the lean ones. Obese adolescents had significantly higher BMI and waist/height ratio than the lean groups. Given that we excluded obese adolescents with type 2 diabetes, all participants had fasting glucose levels within the normal range. However, as expected, obese adolescents had significantly higher fasting insulin concentrations and higher HOMA-IR than the lean adolescents. Groups did not differ in total cholesterol and triglyceride levels but did differ on HDL and LDL levels. Intracranial vault-adjusted brain parenchymal volumes were significantly smaller in obese than in lean subjects (lean = 5.25 ± 10.4, obese = −4.08 ± 19.6; P = .001; please refer to Table 1 for the raw volumes, though for the statistical analyses we used the residuals). Furthermore, obese adolescents had more CSF volumes than lean adolescents, and the difference in intracranial vault-adjusted CSF volumes was also significant (lean = −5.25 ± 10.4, obese = 4.08 ± 19.6; P = .001; please refer to Table 1 for the raw volumes, which suggest that the brain of obese adolescents may have undergone more atrophy since achieving maximal brain volume in early adolescence).
Demographic and endocrine dataa
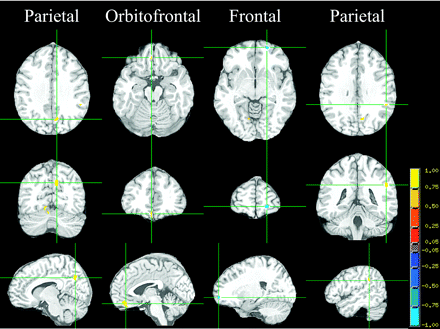
The T1 values appear quite consistent in Fig 1, with no outliers, which would appear as values very different from their neighbors or as gray dots if they fell outside the thresholds used to exclude noise and CSF. This consistency suggests that our measurements are quite precise. The VANCOVA analyses yielded a total of 7 clusters that exceeded the size threshold of at least 100 contiguous significant voxels (P < .005) and an FDR < 0.005. Three of the clusters were located in the cerebellum and were excluded from study because the complex topology of the cerebellum may cause registration problems. Three of the cerebral clusters, located in the OFC and the parietal lobe, represented longer T1s among obese adolescents compared with lean ones (Fig 2 and Tables 2 and 3); the fourth cluster, which was located in the frontal lobe, represented shorter T1s among obese adolescents (Fig 2 and Tables 2 and 3).
T1 map computation. Five native inversion-recovery scans are coregistered to the TI = 675 image, and the T1 maps are generated from the normalized inversion-recovery scans. The T1 map is then skull-stripped and normalized to standard space.
ANCOVA voxelwise analyses. Each column represents the 3 orthogonal orientations (axial, coronal, and sagittal) for the significant T1 difference between groups (analysis controlling for age, MAP, and intracranial vault-residualized brain parenchyma; minimum cluster size, 100 voxels; P < .005; FDR < 0.5%) overlaid on the T1 target image.
Significant clusters demonstrating longer T1 (t value >0) or shorter T1 (t value <0) in obese adolescents compared with lean onesa
T1 value of each significant clustera
Discussion
This study is the first to characterize differences in brain T1 relaxation between lean and obese adolescents. Relative to lean adolescents, obese adolescents had significantly smaller brain parenchyma after controlling for vault volume (P < .001); greater CSF volume (P < .012), suggesting parenchymal atrophy; and longer T1 relaxation times (P < .005) in several regions, including the parietal lobes and the OFC.
Compared with lean adolescents, obese adolescents had both significantly smaller parenchymal brain volume (after accounting for intracranial vault volume) and significantly larger CSF volume. This observation is consistent with evidence that obesity is associated with smaller GM and WM volumes.17⇓–19 This cerebral volume difference could potentially result in a systematic difference in T1 between lean and obese subjects. Size normalization is a process of deforming individual brains systematically so that anatomic landmarks in different subjects have similar spatial coordinates. Size normalization could potentially result in an artifactual T1 difference between groups if there are systematic differences in brain volume. For example, locations in standard space that are pure WM in lean subjects might contain a mix of both WM and GM in obese subjects. The longer T1 of GM could then bias the calculation of average T1 in that volume, even if the voxel is still classified as WM. It is very unlikely that a brain voxel would be categorized as a nonbrain voxel; hence, cerebral volume is less likely to be contaminated by CSF. Therefore, to control for the possibility that certain voxels were erroneously categorized as WM or GM, especially at the margin between the 2 tissues, in our voxelwise T1 comparison between groups, we controlled for intracranial vault−adjusted cerebral parenchyma volumes.
Contrary to our hypothesis, most clusters showed an increase of T1 among obese adolescents. Perhaps this suggests that the developmental trajectory may be delayed by the metabolic problems associated with obesity. This is counter-intuitive because of the known accelerated sexual maturation in this group. However, these findings were not completely uniform because we also found that 1 frontal cluster (see the blue cluster on Fig 2) represented a shorter T1 among the obese group.
A limitation of this work is that the voxelwise methodology may overestimate the significance of intersubject differences. Yet this is not likely because we chose a very conservative threshold for voxel detection (P < .005 and FDR < 0.5%) to overcome this limitation inherent to the voxelwise analysis. In fact, image preprocessing is critical in voxel-based morphometry image analysis; anatomic variation between subjects is treated as if it were noise, to be factored out of the data insofar as possible.20 Furthermore, statistical testing is done on a voxel-by-voxel basis, so Gaussian random field theory is used to correct for multiple comparisons,20 though the FDR of the Gaussian random field can be misleading if there is unexpected signal-intensity inhomogeneity.21 Furthermore, small errors in registration can lead to major errors in voxelwise analyses, in that the method is sensitive to factors that cause nonlinear distortion of base images.22 However, given that we used a very conservative FDR threshold, our voxelwise analysis should be less vulnerable to a repeated-measures problem, and it is not likely that any of the clusters of different T1s among the obese group are false-positive due to the analysis method.
Brain maturation continues through adolescence and well into adulthood.23 For example, Paus et al24 reported an age-related increase in WM attenuation in the fiber tracts of the putative corticospinal and frontotemporal pathways. Moreover, increased myelination and synaptic pruning associated with loss of GM attenuation were observed in the dorsal parietal and frontal cortices.25,26 Although little is known about the impact of obesity on brain maturation, obesity is known to affect body maturation. For instance, obese children achieve faster sexual maturation, as measured by estradiol levels or bone age, than do their lean counterparts.27 Therefore, despite lean and obese participants all being Tanner sexual development stage 4 or greater, it is possible that the longer brain T1 that we observed in the OFC and parietal lobes of obese adolescents, coupled with their smaller brain parenchymal sizes, represents a delay in the developmental trajectory of obese adolescents.
Whitford et al28 showed evidence of GM synaptic pruning and increased myelination that occurs during healthy adolescence, with changes primarily in the frontal and parietal lobes. Consequently, our results of mostly T1 lengthening may suggest that obese adolescents showed a different neurodevelopmental trajectory and that brain areas such as the OFC may be particularly affected. This finding may be consistent with the lower OFC volume, the behavioral disinhibition, and the lower performance in cognitive tests that require intact OFC function described in obese adolescents relative to well-matched lean adolescents.9 This longer T1, which goes against our hypothesis, could indicate that the metabolic dysregulation associated with obesity may be associated with delayed brain maturation in the obese group.
However, although our results predominantly indicated longer T1 in obese adolescents, some brain regions showed our hypothesized result. When we used less conservative statistics (P < .01), we observed many small clusters that represent a shortening of T1 among obese adolescents. In addition, we also had a significant cluster in the frontal lobe showing shorter T1 in the obese group even with the conservative statistics. This shortening could be interpreted as accelerated maturation, but this is not likely given that most significant clusters were in the opposite direction (namely a lengthened T1 in obesity) and given the evidence of reductions in cognitive performance, in brain volumes, and DTI-based microstructural integrity. Because shortening of brain T1 is associated with lipid deposition, the areas of shortened T1 may suggest that the brains of obese adolescents contain more lipid than the brains of lean adolescents. Although we have no direct evidence that increased brain lipid explains the shorter T1 among obese adolescents, some indirect evidence is consistent with this hypothesis. Researchers using the positron-emitting free fatty acid ligands 11C-palmitate and [18F]fluoro-6-thia-heptadecanoic acid found that fatty acids are taken up and accumulated in the brain more by obese individuals with metabolic syndrome than by lean subjects and that this difference is partly reversed by weight reduction among obese individuals.29 To test this hypothesis directly, we propose to study the relationship between obesity and brain T1 in diet-induced obese rats and their lean counterparts and then to carefully measure cerebral lipid content.
Brain assessment in obesity has predominantly focused on overt structural changes (whole brain volume and GM and WM volume)17⇓–19; to the best of our knowledge, there are no previous in vivo or postmortem human studies that assess T1 as a marker of brain maturation in obese compared with lean individuals. We provide intriguing preliminary evidence to support the hypothesis that relative to lean adolescents, obese adolescents may have a delayed neurodevelopmental trajectory or metabolic changes in the brain during adolescence.
Conclusions
This is the first study to assess brain T1 in obese and lean adolescents. We found significant T1 changes among obese adolescents, which suggest that obesity may have an impact on brain development or may trigger metabolic changes in the central nervous system. It is unclear if these changes persist into adulthood or whether they show that obese subjects follow a different developmental trajectory during adolescence.
Footnotes
-
This work was supported by a grant from the National Institutes of Health DK083537 and in part by grant 1UL1RR029893 from the National Center for Research Resources.
-
Disclosures: Wai H. Tsui, UNRELATED: Patents (planned, pending or issued): Abiant Imaging, US Patent No. 7787671. R. Grant Steen, UNRELATED: Consultancy: MediCC, MediCC is my company and I consult for many clients in the biomedical sciences. However, I received no money from Dr Convit or any of the other authors or institutions affiliated with the work under consideration by the American Journal of Neuroradiology; Payment for Manuscript Preparation: MediCC, I received no money in relation to the manuscript under consideration by the American Journal of Neuroradiology; Payment for Development of Educational Presentations: MediCC, I received no money in relation to the manuscript under consideration by the American Journal of Neuroradiology. Antonio Convit, RELATED: Grant: National Institutes of Health. (money to New York University School of Medicine)
Indicates open access to non-subscribers at www.ajnr.org
References
- Received April 11, 2011.
- Accepted after revision April 19, 2011.
- © 2011 by American Journal of Neuroradiology