Abstract
BACKGROUND AND PURPOSE: Stereotactic radiosurgery is known to control 85%–95% of intracranial metastatic lesions during a median survival of 6–8 months. However, with the advent of newer systemic cancer therapies, survival is improving; this change mandates a longitudinal quantitative analysis of the radiographic response of brain metastases to radiosurgery.
MATERIALS AND METHODS: MR imaging of 516 metastases in 120 patients treated with GK-SRS from June 2006 to December 2009 was retrospectively reviewed. Lesion volume at initial treatment and each follow-up was calculated by using the following formula: length × width × height / 2. Volume changes were correlated with patient demographics, histopathology, and radiation treatment variables.
RESULTS: Thirty-two percent of lesions increased in volume following radiosurgery. Clinically, this translated into 54% of patients having ≥1 of their lesions increase in size. This increase begins at 6 weeks and can last beyond 15 months' post-SRS. Male sex (P = .002), mean voxel dose <37 Gy (P = .009), and initial treatment volume >500 mm3 (P < .001) are associated with posttreatment increases in tumor size. Median survival following radiosurgery was 9.5 months for patients with all lesions exhibiting stable/decreased volumes, >18.4 months for patients with all lesions exhibiting increased volumes, and 16.4 months for patients with mixed lesional responses.
CONCLUSIONS: Most metastatic lesions are stable or smaller in size during the first 36 months post-SRS. However, a transient increase in volume is seen in approximately one-third of lesions. Sex, treatment dose, initial lesion size, and histopathology all correlate with variations in lesion volume post-SRS. The longer the patient survives, the more likely an increase in lesion size will be seen on follow-up imaging.
Abbreviations
- DWI
- diffusion-weighted imaging
- FDG-PET
- fluorodeoxyglucose–positron-emission tomography
- FLAIR
- fluid-attenuated inversion recovery
- GK
- Gamma Knife
- MPRAGE
- magnetization-prepared rapid acquisition of gradient echo
- SRS
- stereotactic radiosurgery
- WBRT
- whole-brain radiation therapy
The incidence of intracranial metastatic disease is estimated to be >170 000 new cases per year in the United States.1 This number is projected to rise with an aging population, an increased prevalence of individuals living with cancer due to improved systemic control, and improvements in imaging quality and accessibility.1 The use of SRS for treatment of metastatic intracranial disease has grown in popularity due to its increasing ease of use, the avoidance of cognitive side effects related to WBRT, its ease of scheduling between cycles of chemotherapy, and its inclusion as a treatment option in clinical trials.
The effectiveness of SRS is well-documented.2–5 The average survival of patients with SRS-treated brain metastases is 6–8 months. Within this timeframe, studies have shown SRS to achieve a 90% radiographic lesional control rate, defined as lesions with stable or decreasing size.6–10 However, transient increases in the size of up to 12% of lesions have been reported, and 9% of lesions at 4-year follow-up can be larger than they were at the time of treatment.6,8 Lesion control rates are reported to vary by time since radiosurgery, pathology, lesion size, and treatment dose.10–14 However, no single study has looked at all of these factors during serial follow-up imaging.
In clinical practice, increasing radiographic lesion size post-SRS raises the question of treatment failure versus radiation injury, resulting in difficulty with patient management. Many studies have reported variable degrees of diagnostic sensitivity and specificity using various MR imaging sequences and positron-emission tomography imaging techniques, but consensus does not exist.15,16 Historically, at our center, patients whose serial MR images post-SRS demonstrated both progressively increasing lesion size and increasing surrounding FLAIR signal-intensity abnormality were sent for brain FDG-PET and/or proton MR spectroscopy and DWI. If any of these scan results were positive or conflicting, a surgical biopsy or resection was performed. However, in almost all (22 of 23) cases, regardless of imaging findings, growing lesion histopathology has been consistent with radiation necrosis, without evidence of tumor regrowth.
This finding resulted in a change in practice at our institution so that only patients with focal symptoms associated with an increase in lesional size post-SRS undergo surgical decompression. This has allowed us to gather longitudinal data regarding lesional changes post-SRS to understand the MR imaging response of SRS-treated metastatic lesions. Specifically, we have retrospectively reviewed the treatment and long-term follow-up imaging in patients with SRS-treated intracranial metastatic disease for the following purposes: 1) to document the change in lesion size with time, 2) to determine if specific clinical, lesional, or treatment factors correlate with change in lesion size, and 3) to determine if changes in lesion size correlate with survival. A more complete understanding of the variations seen in post-SRS imaging will improve the accuracy of scan interpretation.
Materials and Methods
Patient Data
We performed an Institutional Review Board−approved retrospective review of the medical records of 120 consecutive patients with 516 brain metastases who were treated with GK-SRS (Leksell Gamma Knife, model 4C, GammaPlan 5.3; Elekta Instruments, Stockholm, Sweden) at a single large academic medical center from June 1, 2006, to December 30, 2009. Patients were included in this study if they were >18 years old, had at least 1 posttreatment MR imaging study (at >6 weeks post-SRS), and had appropriate supporting clinical data. Of note, if a patient developed new lesions after initial treatment, these new lesions were also treated and included in the study. All patients gave their informed consent before inclusion. Patients were excluded if SRS was performed on the basis of CT imaging or SRS was performed for consolidation to a surgical resection bed only. Additionally, data collection was terminated early in 10 lesions that required salvage surgery due to symptomatic local failure and 4 lesions due to substantial intralesional bleeding. Follow-up scans were obtained at the discretion of the treating clinician. For purposes of analysis, scans were grouped into 6-week intervals and evaluated up to 36 months post-SRS. The SRS dose delivered to the tumor margin ranged from 18 to 24 Gy prescribed to the 40%–70% isodose surface. Radioresistant tumors (melanoma, renal) received a median marginal dose of 23.7 Gy (range, 20–24 Gy), and radiosensitive tumors (lung, breast, colon, other) received a median marginal dose of 21.3 Gy (range, 18–24 Gy). All patients were weaned off steroids within 4 weeks of completing SRS treatment. We accessed the Connecticut Tumor Registry (http://www.cancer-rates.info/ct/index.php) and the Social Security Administration Death Master File (www.ssdmf.com) to obtain dates of all patient deaths that occurred by June 30, 2010.
MR Imaging
Initial MR imaging was performed on a 1.5T scanner (Magnetom Avanto; Siemens, Erlangen, Germany) with a 12-channel phased-array head coil. 3D MPRAGE, T1-weighted axial images of the whole brain were obtained, without magnetization transfer, following intravenous administration of single-dose gadolinium contrast (0.1 mmol/kg). Gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany) was used for patients with normal renal function, while gadoteridol (ProHance; Bracco, Milan, Italy) was used for patients with impaired renal function. Imaging parameters were as follows: TR, 1900 ms; TE, 2.66 ms; TI, 1100 ms; flip angle, 15°; FOV, 256 × 256 mm; matrix, 256 × 256; section thickness, 1 mm; averaging, 1; sections per slab, 224; and acquisition time, 5 minutes 15 seconds. The generalized autocalibrating partially parallel acquisition algorithm was applied with a reduction factor of 2. Sagittal and coronal reformats were subsequently obtained. All patients were followed with 1.5T, 3D MPRAGE, T1-weighted single-dose gadolinium-enhanced MR imaging without magnetization transfer in the axial, coronal, and sagittal planes. Specific protocol parameters for follow-up studies varied because many patients preferred to have their follow-up imaging performed locally.
Volumetric Analysis
The maximal diameter of the enhancing lesion was measured in 3 orthogonal planes at the time of treatment. Lesion volume was calculated according to the following formula: volume = length × width × height/2.17 The calculated volume of each treated lesion was measured on each follow-up MR imaging until the last available scan. Each follow-up lesion volume was then compared with the pretreatment (initial) lesion volume (for division of lesions into group A versus group B or C, see below). For group B and C lesions, each follow-up lesion volume was then compared with the calculated tumor volumes from any prior follow-up scans (for division of lesions into group B versus C, see below).
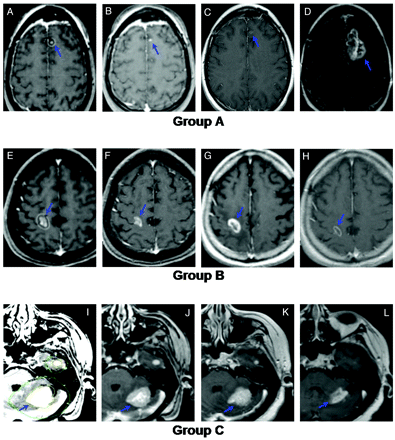
A stable lesion was defined as any change in volume of <20%. An increasing lesion was therefore defined by a volume of >120% of the comparison volume, and a decreasing lesion was defined by a volume of <80% of the comparison volume. Three lesion-based groups were then defined for subsequent analyses. Group A was defined as tumors that had, at any time, an increase in volume of >20% above the pretreatment initial lesion volume. Group B comprised lesions whose volumes fluctuated during follow-up (ie, they initially decreased and then subsequently increased) but never exceeded 20% above the pretreatment initial lesion volume. Group C contained tumors that remained consistently stable or regressed after SRS, (ie, they never increased throughout the follow-up period, Fig 1).
Examples illustrating the study definitions of groups A, B, and C. A−D, Representative images from a group A lesion (increased in follow-up to a volume >120% of initial size). A, Gamma knife treatment plan for a 36-year-old woman nonsmoker with non-small cell lung carcinoma. The lesion was treated with 18 Gy to the 50% isodose line. B, Initial MR image. C, Six-month follow-up MR image. D, Twelve-month follow-up MR image. E−H, Representative images from a group B lesion (size fluctuated throughout follow-up but never increased beyond 120% of the initial size). E, Gamma Knife treatment plan for a 75-year-old man with colorectal adenocarcinoma. The lesion was treated with 20 Gy to the 50% isodose line. F, Initial MR image. G, Three-month follow-up MR image. H, Six-month follow-up MR image. I–L, Representative images from a group C lesion (remained stable or decreased in size throughout follow-up). I, Gamma Knife treatment plan for a 76-year-old woman with 2 metastatic breast cancer lesions. Both lesions were treated with 20 Gy to the 50% isodose line. J, Initial MR image. K, Six-month follow-up MR image. L, Twelve-month follow-up MR image.
Statistical Analyses
Statistical analyses were performed by using STATA (Version 9.0, StataCorp, College Station, Texas). Descriptive statistics were used to evaluate the incidence of volume change, as well as to measure the longitudinal percentage change in tumor volume. We used the Fisher exact test (for categoric variables) and the Wilcoxon signed-rank test (for continuous variables) as well as multivariate logistic regression, to determine which clinical factors (mean, maximum, and minimum radiosurgery doses; initial treatment volume; histopathology; age; sex; race; the use of systemic therapy; and pre-SRS WBRT) correlated with change in lesion size. Additionally, we examined a variety of cutoff points (quartiles and deciles) for further investigation of continuous variables. Last, Kaplan-Meier analyses were performed to compare survival in patients with lesions that all increased in size versus patients with lesions that all remained stable or decreased in size versus patients with mixed lesional responses.
Results
One hundred sixty-three patients with intracerebral metastases underwent GK-SRS between June 2006 and December 2009. Forty-three patients were excluded from this study due to lack of follow-up imaging (28 patients), age <18 years (2 patients), GK-SRS treatment based on CT imaging (5 patients), and GK-SRS delivered to postoperative resection cavities (8 patients). Therefore, the study cohort consisted of 120 patients: 53 men and 67 women with a median age of 59.7 years (Table 1). The median number of follow-up scans obtained per patient was 4 (range, 1–14). A total of 516 metastases were treated. Thirty-four patients had single metastases treated, 42 had 2–4 metastases treated, 34 had 5–10 metastases treated, and 10 patients had >10 metastases treated (range, 1–22). Median initial lesion volume was 126.8 mm3 (range, 2–26,775 mm3). Thirty-four percent of the metastases treated (in 29% of patients) had radioresistant histologies (melanoma, renal), while 66% of the metastases treated (in 71% of patients) had radiosensitive histologies (lung, breast, colon, other). At the conclusion of the study, 94 of the 120 patients had died (78%). Median survival for deceased patients was 19.5 months (range, 1.4–44.7 months). Median follow-up for those still alive was 24.1 months (range, 17.4–48.5 months).
Patient and lesional characteristics
Descriptive Tumor Volume Analysis
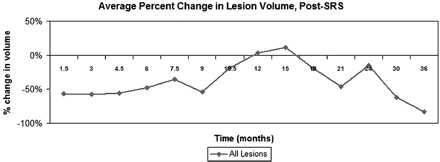
At each follow-up time point, 70%–95% of metastases were stable or decreased in volume compared with their initial treatment volume. At 6 weeks post-SRS, lesions decreased by an average of 56.5%, and, as a group, remained relatively stable until 9 months post-SRS (Table 2). Lesion size then increased on the 10.5-, 12-, and 15-month follow-up MR images, so that the average lesion size at 10.5 months was only 18.7% smaller than initial treatment volume. By 12 months, the average lesion size was 3.6% larger than the initial treatment volume, and at 15 months, the average lesion size was 11.6% larger than initial treatment volume. By 18 months, average lesion size had decreased again to 20.1% smaller than initial treatment volume, and by 21 months, the average volumes had returned to early posttreatment values (Table 2 and Fig 2).
Average percentage change in lesion volume with time, relative to initial treatment volume
Average change in lesional size with time, relative to initial treatment volume, all lesions. Lesions decreased or remained stable in size for the first 9 months post-SRS. Subsequently, they increased in size until approximately 18 months post-SRS, at which point they began to decrease in size once again.
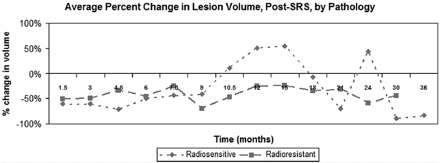
If we divided tumors by histopathology, metastases from radiosensitive primary tumors (lung, breast, colon, other) were more likely to increase during the first 12–18 months post-SRS than metastases from radioresistant tumors (melanoma, renal) (Fig 3). However, this difference did not extend, consistently, beyond 18 months.
Average change in lesional size with time, relative to the initial treatment volume, separated by histopathology (radiosensitive: lung, breast, colon, other; radioresistant: melanoma, renal). Radiosensitive tumors were more likely to increase in size during the first 12–18 months post-SRS than radioresistant tumors. However, this difference did not extend, consistently, beyond 18 months.
All lesions were then separated into groups A, B, and C as described in the “Materials and Methods” section (Fig 1). Of the 516 lesions, 81 (15.7%) fulfilled the criteria for group A; 83 (16.1%) for group B; and 352 (68.2%) for group C. Of the 81 lesions in group A, the time post-SRS at which they began to increase in size ranged from 6 weeks to 21 months. Twenty-five of these 81 lesions (30.9%) had already increased in size at 6-week follow-up, and another 29 lesions (35.7%) began to increase in volume 3–6 months post-SRS (Table 3). Of the 83 lesions in group B, 67 of these lesions (82.5%) began increasing in volume 3–6 months post-SRS; the overall range in time to volume increase was 3–21 months.
Timing of lesional increases in size (percentage of total group A or B lesions beginning to increase in size at a given timepoint)
Further analysis revealed that not all lesions in a single patient would uniformly increase or decrease in size post-SRS. Fifty-five of the 120 patients had lesions that all remained stable or decreased in size post-SRS (ie, all group C lesions). Of the remaining 65 patients, 11 had lesions that all increased in size (all group A lesions); the remaining 54 patients had mixed lesional responses (combination of groups A, B, and C) (Table 4).
Variability in lesional response categorized by patienta
Univariate and Multivariate Analyses
On univariate analysis, sex (P = .003), histopathology (P = .008), mean treatment voxel dose (P = .013), minimum treatment dose (P = .002), and initial treatment volume (P < .001) were significantly different among groups A, B, and C. Higher marginal SRS doses (>22 Gy) correlated with a decreased likelihood that lesions would increase in size (P = .037). Of note, there was a statistically significant difference between the median SRS dose prescribed to the edge of radiosensitive (breast, lung, colon, other: 21.3 Gy) versus radioresistant (melanoma, renal: 23.7 Gy) lesions (P < .001). Furthermore, the larger the initial treatment volume was, the more likely the lesion would increase, with the most significant cutoff occurring at an initial lesion volume of ∼500 mm3.
Multivariate analysis identified male sex (P = .002), mean voxel dose <37 Gy (P = .009), and larger initial treatment volumes (P < .001) as factors that independently correlated with an increase in lesion volume. Female sex (P = .002), initial treatment volume <25 mm3 (P < .001), non-small cell lung cancer (P < .001), breast cancer (P = .023), and renal cell carcinoma (P < .001) independently correlated with stable or decreased tumor volumes. Age, race, use of systemic therapy, all other histopathologies, and pre-SRS WBRT failed to demonstrate statistical significance.
Kaplan-Meier Analyses
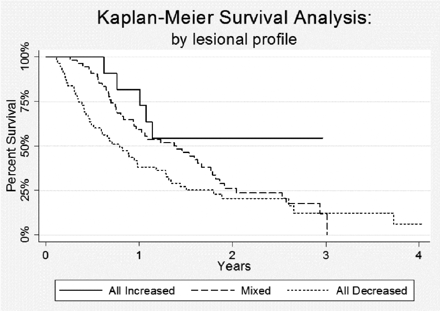
To understand the relationship of lesional volume change to survival, we performed Kaplan-Meier analyses to compare survival in patients with all lesions increased (group A) with that of patients with all lesions stable or decreased (group C) and that of patients with mixed lesional responses (any combination of groups A, B, and C lesions) (Fig 4). Median survival was 9.5 months for patients in whom all SRS-treated lesions decreased or remained stable in size, compared with 16.4 months for patients whose lesions had mixed responses to SRS. For patients in whom all lesions increased in size, median survival was not yet reached at the end of the study period. Specifically, at last follow-up, 6 of the 11 patients (55%) with all increased lesions were still alive (median follow-up = 18.4 months) compared with only 9 of 55 patients (16%) with all stable or decreased lesions and 11 of 54 patients (20%) with mixed lesional responses.
Kaplan-Meier survival curve, by lesional profile, demonstrates that patients with lesions that all increased in size following SRS had significantly improved survival (P = .035).
Surgical Histopathology
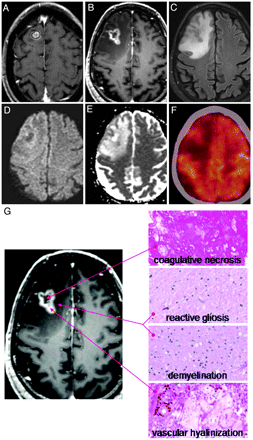
Of note, 10 lesions required salvage surgery due to focal neurologic deficits caused by increased lesional size and surrounding edema. Histopathology in all cases demonstrated a significant inflammatory infiltrate with central necrosis, consistent with radiation-induced necrosis (Fig 5).
Example of radiologic and histopathologic changes in enlarging lesions. A, Gamma Knife treatment plan for a 56-year-old man with metastatic melanoma. The lesion was treated with 22 Gy to the 50% isodose line. B, Twelve-month follow-up T1 postcontrast MR imaging. C, Twelve-month FLAIR image. D and E, Twelve-month DWI/apparent diffusion coefficient images, respectively. F, Twelve-month FDG-PET image. G, Histopathology from stereotactic image-guided biopsies of the lesion. Specimens from the central, T1 hypointense portion of the lesion demonstrate coagulative necrosis. Specimens from the peripheral, T1 hypointense portion of the lesion demonstrate reactive gliosis and demyelination. Specimens from the T1 contrast-enhancing portion of the lesion demonstrate vascular hyalinization. This constellation of histopathologic findings suggests a diagnosis of radiation-induced changes.
Discussion
The incidence of metastases increasing in size post-SRS is not well-known. However, an increase in lesion size on follow-up MR imaging not only creates management dilemmas but is also anxiety-provoking for patients and their referring physicians, who interpret any increase in lesion size as cancer regrowth. Therefore, to understand the natural history of post-SRS MR imaging changes, we followed all SRS-treated lesions with serial MR imaging until the patient became symptomatic or died.
In this study, we found that approximately one-third of SRS-treated lesions increased in size during follow-up. Consistent with previous reports,10 this study found that increasing initial treatment volume is associated with a higher probability of a post-SRS increase in lesion volume. What has not been previously reported, to our knowledge, is that male sex and mean voxel dose <37 Gy are also associated with a higher probability of a post-SRS increase in lesion size. With regard to timing of lesion growth, most lesions increase in size 3–6 months post-SRS. However, this enlargement can start as early as 6 weeks post-SRS and may not reach peak volume until 15 months post-SRS.
Most interesting, 9% of patients had SRS-treated lesions that all increased to >120% of their original treatment size, 45% of patients had mixed lesional responses, and only 46% of patients had lesions that all remained stable or decreased as expected. Therefore, more than one-half of patients, at some point during follow-up, were told that at least 1 of their SRS-treated lesions had increased in size. Despite this increase, only 10 of 120 patients (8%) in this study required salvage surgery. Therefore, the vast majority of lesional volume increases are asymptomatic and require only observation.
Lastly, this study reports a survival difference based on lesional response to SRS. For those patients in whom all lesions increased in size, median survival was >18.4 months, compared with 16.4 months for patients whose lesions showed mixed responses and 9.5 months for patients whose lesions all remained stable or decreased in size. Initially, this finding seems counterintuitive. Traditionally, lesional growth is thought to be tumor recurrence and would, therefore, be associated with shortened survival. The exact opposite was found in this study, and we propose an alternative explanation. Not only do we have clear examples of lesions that initially decreased and subsequently increased in size post-SRS, but we have also found that the histopathologic diagnosis in these enlarging lesions is most often consistent with inflammation and necrosis. Histologically, radiation-induced injury has been well-described as areas of necrosis surrounded by a robust inflammatory cell infiltrate that may also contain some tumor cells.18,19 Therefore, the post-SRS lesional growth seen in this study may not represent tumor regrowth at all but rather a brisk reactive immune response that is related to or causing apparent lesional growth on MR imaging.
Current literature suggests that immunomodulation can affect cancer response to chemotherapeutics; specifically, that an exaggerated immune response results in improved survival and control of cancer.20,21 In particular, immunotherapy has been used extensively for the treatment of systemic melanoma. Our study contains an unusual predominance of patients with melanoma (20%) with an unusually long median survival of 13.7 months. Therefore, we propose that in some patients, a strong immune system is better able to fight systemic cancer (therefore prolonging survival) as well as respond to high-dose radiation with an exaggerated inflammatory response, leading to a larger enhancing cerebral lesion, thus providing a single explanation for the improved survival in patients with lesions that increase in size post-SRS. Prospective assays for a systemic marker of immunocompetence as well as biopsies at the time of lesion growth could help determine the mechanistic validity of this proposed explanation. Alternatively, a simpler explanation may be that the longer the patient survives, the higher the chance of developing adverse effects to radiation therapy. This explanation seems less likely, however, given that approximately 30% of lesions in group A started to increase in size within 6 weeks of SRS.
Shortcomings of this study include significant heterogeneity in the types of chemotherapeutic agents that were used at the time of each follow-up MR imaging, limiting our ability to assess the effects of individual chemotherapeutic agents. This is especially true with regard to antiangiogenic agents such as bevacizumab, which have been shown to affect vessel permeability and apparent lesion size. Due to the large variety of chemotherapeutic and immunologic agents used in this patient population, no correlation was identifiable between the agents used and radiographic changes.
Another possible shortcoming may be the method used to measure lesion volume. Almost all of the previous studies analyzing lesion-volume changes have used specialized volumetric software. However, software that is capable of accurately calculating lesion volume through accurate segmentation is not available to most oncologists and radiologists at this time, and reporting of lesion volume is not standardized. One calculates the volume of a spheroidal lesion with the following formula: volume = (4/3)πr3. For an ellipsoid, the individual orthogonal radii would be substituted for the radius of the sphere. However, post-SRS, metastatic tumors often deviate from simple geometric shapes. A method of lesion measurement that more closely approximates clinical practice documents the largest diameters in 3 orthogonal planes and applies the formula: volume = length × width × height/2. While this method has not been validated for tumors, it has previously been used and validated in the intracerebral hematoma literature in the setting of even irregularly shaped lesions.17 To validate our findings, a follow-up study would need to be performed with volumetric software. This study allowed a generous 20% margin in lesion-volume change to account for possible errors in this methodology, and the same calculation error would have been carried equally throughout the study.
Lastly, this study was performed retrospectively, and not all data points were available on all patients. While our referral sources, historically, have been reliable about sending patients back for consultation when lesions increase in size, it is possible that bias was introduced by data omission.
Conclusions
Patients with intracranial metastases are surviving longer. Given the success and increasing availability of SRS for the treatment of brain metastases as well as the increased willingness of the oncology community to accept SRS as a first-line treatment for multiple intracranial metastases, it is important to recognize the variability in lesion response to SRS, identify factors that correlate with lesion response, and examine the implications of this variability. This study suggests that one- third of SRS-treated lesions will increase in size on follow-up imaging and more than one-half of patients will have at least 1 lesion increase in size on follow-up. However, most lesional increases are both transient and asymptomatic and can be managed conservatively. Lastly, patients who demonstrate prolonged asymptomatic increases in lesion size post-SRS appear to have a survival advantage compared with those whose lesions have mixed responses or decrease in size.
Acknowledgments
We thank Courtney Rubin, Elizabeth Pon, and Judith Hess for assistance in data collection.
Footnotes
-
T.R.P. and B.J.M. contributed equally to this work.
-
Paper previously presented in part at: The 15th International Meeting of the Leksell Gamma Knife Society, May 16–20, 2010; Athens, Greece.
References
- Received December 29, 2010.
- Accepted after revision March 17, 2011.
- © 2011 by American Journal of Neuroradiology