Abstract
BACKGROUND AND PURPOSE: Patients harboring nongiant cerebral aneurysms may rarely present with an ischemic infarct distal to the aneurysm. The aim of this case series was to report clinical and radiologic characteristics of these patients, their management, and outcome.
MATERIALS AND METHODS: We undertook a single-center retrospective analysis of consecutive patients admitted during an 8-year period with an acute ischemic stroke revealing an unruptured nongiant (<25 mm) sacciform intracranial aneurysm. Clinical, radiologic, therapeutic, and follow-up data were analyzed.
RESULTS: Nine patients were included. The mean size of aneurysms was 9.6 ± 6 mm, and 5 were partially or totally thrombosed. Two patients had a fatal SAH within 3 days after stroke-symptom onset, whereas asymptomatic meningeal bleeding was diagnosed or suspected in 2 others. Most of the patients with unthrombosed aneurysms were successfully treated by endovascular coiling in the acute phase. Thrombosed aneurysms were usually treated with antithrombotics, and most recanalized secondarily, requiring endovascular treatment or surgical obliteration. No recurrence of an ischemic event or SAH was observed during the 31 ± 12 months of follow-up (from 4 to 53 months).
CONCLUSIONS: In this single-center series, the frequency of early SAH in patients with ischemic stroke distal to an unruptured intracranial aneurysm was high. Acute management should be undertaken with care regarding antithrombotic use, and early endovascular coiling should be considered.
Abbreviations
- MCA
- middle cerebral artery
- mRS
- modified Rankin Scale
- NIHSS
- National Institutes of Health Stroke Scale
- PCA
- posterior cerebral artery
- PICA
- posterior inferior cerebellar artery
- SAH
- subarachnoid hemorrhage
Ischemic stroke and transient ischemic attack are rare but well-documented presentations of intracranial aneurysms.1 While being more common in giant thrombosed aneurysms, this kind of complication has also been described in small (<10 mm) and large (<25 mm) aneurysms.2–10 Cerebral ischemia results from an emboli originating within the aneurysmal sac or from the extension of an aneurysmal thrombosis to the parent artery lumen.11,12 Although the clinical course of aneurysms revealed by an ischemic attack appears to be benign,2–7 a few reports emphasized that an SAH may occur subsequently, soon after the ischemic event.8,9,13,14 This finding suggests that an aneurysmal wall remodeling may lead to intrasaccular thrombosis and clot migration and secondary rupture.14 The risk of bleeding raises the issue of the best management of those patients with acute ischemic stroke. We will report our experience of 9 consecutive patients with ischemic stroke with nongiant intracranial aneurysms. On the basis of the high rate of early SAH observed in our series, we suggest that acute management should be undertaken with great care regarding the use of antithrombotics, and the early treatment of the aneurysm should be considered.
Materials and Methods
Patients admitted in our stroke unit from October 2001 to October 2009 with an ischemic stroke and an unruptured <25-mm sacciform intracranial aneurysm were selected from our data base. To be included, patients should have had the following: 1) an ischemic event in a distribution distal to a nongiant intracranial aneurysm; 2) no history of intracranial aneurysm and no symptoms of SAH such as thunderclap headache before stroke symptom onset; 3) CT scans, MR imaging studies, and 4-vessel angiography performed, ruling out vasospasm secondary to SAH, a large infundibulum of a lenticulostriate artery, or features of intracranial dissection (defined as fusiform aneurysm, double lumen, intramural hematoma, stenosis with or without dilation); and 4) an extensive work-up excluding other causes of infarction (standard blood tests, electrocardiography, and echocardiographic and cervical artery sonographic studies).
CSF obtained from lumbar puncture between 12 hours and 10 days after stroke onset was analyzed for red and white blood cell counts, the presence of xanthochromia, and the detection of bilirubin by using spectrophotometry, in accordance with published guidelines.15–17 The demographics, clinical presentation, site, size and management of the aneurysm, and data regarding the use of antithrombotics were collected. Clinical outcome by using the mRS (0, no symptoms at all; 1, no significant disability despite symptoms; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; 6, death)18 and radiologic follow-up data were recorded between 2 and 4 months after presentation and the last visit for each patient.
Results
Nine patients fulfilled our selection criteria (5 women; mean age, 50.8 ± 7.4 years; range, 38–63 years). These patients represented 0.2% of all patients admitted in our stroke unit during the 8-year study period and 1.4% of the population of patients with symptomatic intracranial aneurysms managed in our hospital. Baseline characteristics, acute management, aneurysm pattern, and patient outcome are presented in On-line Tables 1 and 2. All presented with an ischemic stroke distal to a small or large sacciform intracranial aneurysm. Infarctions were located in the MCA territory in 4 and in the posterior circulation in 5. Work-up did not identify atherothrombotic or cardioembolic origin or small vessel disease. Headache or orbital pain or both were noted at presentation in 2 patients and appeared at 24 hours in another. No patient had neck stiffness or meningeal syndrome, and there was no sign of SAH on the initial CT scan or MR imaging. Aneurysms were <25 mm in their greatest diameter, with a mean size of 9.6 ± 6 mm (range, 3–20 mm).
Digital angiography and MR imaging studies showed partial or complete aneurysm thrombosis in 5, whereas the aneurysmal sac was free of thrombus in the others. The parent artery was occluded or narrowed in 4 (Fig 1A, -B). Lumbar puncture was performed in 5 patients from 24 hours to 10 days after the onset of symptoms. Four had a spectrophotometry analysis, which depicted an unexpected and asymptomatic SAH in 1 (patient 7). In the last one (patient 2), lumbar puncture suggested an SAH with an increased red cell count, but there was no spectrophotometry to confirm this suggestion. Two patients (patients 1 and 5) had a severe and acute headache during hospitalization, and their conditions rapidly deteriorated with a loss of consciousness 2 and 3 days after their first ischemic symptoms, revealing a massive SAH (Fig 2A−C). Both died a few days later.
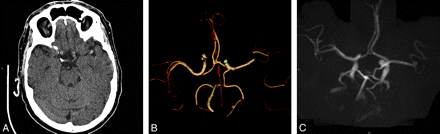
Thrombosis of an intracranial aneurysm and parent artery with early recanalization (case 9). A 63-year-old man had acute aphasia and right hemiparesis (NIHSS score, 21). A, Plain CT scan 2 hours after stroke onset shows a left MCA hyperattenuation, and the presence of a berry aneurysm is suspected on the apparent enlargement of the artery. B, Volume-rendering of CT angiography revealed a left M1-M2 occlusion, and a thrombosed left MCA aneurysm is not detectable. Intravenous thrombolysis was not performed, and after lumbar puncture excluding a meningeal hemorrhage, he was treated with aspirin, 250 mg/day. C, Time-of-flight MR angiography performed the day after shows recanalization of the left MCA aneurysm. He was discharged and sent home on day 8 with persistent aphasia (NIHSS score, 8), and surgical obliteration (clipping) of his aneurysm was performed 6 weeks later (aneurysm anatomy did not allow an intra-arterial procedure).
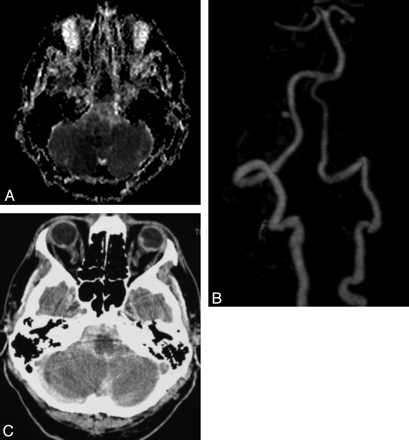
Early SAH after ischemic stroke distal to a PICA aneurysm (case 5). A 53-year-old man presented with sudden onset of vertigo, unsteady gait, and right cerebellar syndrome. Findings of CT performed 6 hours after onset were normal, and aspirin, 250 mg daily, was started. A, Apparent diffusion coefficient cartography of a diffusion-weighted sequence 48 hours later shows diffusion restriction on the right PICA territory. B, Maximum intensity projection of MR contrast angiography shows perfect vertebral and basilar artery patency and a right proximal 4 × 3 mm berry PICA aneurysm, with occlusion of the PICA beyond the aneurysm. During MR imaging, he suddenly complained of severe headache and had rapid deterioration of consciousness, requiring intubation. C, Plain CT scan at the pontocerebellar angle level shows massive SAH. He was transferred into an intensive care unit, where he died a few hours later.
Antithrombotics were used on admission in 4 patients (aspirin in 1, heparin in 3) and within the first week in 6. Four patients had an unthrombosed aneurysm; 3 were successfully treated during the acute stage by endovascular coiling and the last one died early from SAH.
The mean length of follow-up in surviving patients was 31 ± 17 months (from 4 to 53 months). The overall prognosis was excellent for these patients (mRS, 0–2 at 3–4 months). No recurrence of ischemic stroke or SAH was observed. One patient with early aneurysm embolization showed partial recanalization of the aneurysm on imaging at 4 months, and the aneurysm was then successfully re-embolized (patient 8). Three of the 4 patients with complete aneurysm thrombosis showed partial or complete reopening at the first follow-up imaging (2 days to 4 months, Fig 1C). The aneurysm of last patient who also had parent vessel occlusion did not recanalize during follow-up. For those whose aneurysms secondarily recanalized, 2 were treated by endovascular embolization or surgical obliteration and the other refused any invasive treatment. Four patients remained on long-term antiplatelet therapy; 3 had no treatment with antithrombotics.
Discussion
In our series of ischemic strokes revealing unruptured intracranial aneurysm, an early SAH occurred in 2 patients leading to death, diagnosed by CSF spectrophotometry in 1 and suspected by CSF analysis in another. This complication was excluded in 3 by using CSF spectrophotometry and could not be ruled out in the last 2 patients (no lumbar puncture). To our knowledge, this consecutive series is the first to report such a high frequency (at least one-third) of early subarachnoid bleeding in the context of ischemic stroke distal to a small or large unruptured intracranial aneurysm.
Several case series have reported cerebral ischemia as a presenting feature of a nongiant unruptured intracranial aneurysm,2–10 but there are very few reports describing such a symptom as a warning sign of subsequent aneurysmal rupture.8,9,13,14,19
Our study has several practical consequences. First, antithrombotics and fibrinolytics and also intra-arterial therapies should be used with caution for acute ischemic stroke that occurs distal to an intracranial aneurysm because of the risk of bleeding. The risk may be particularly difficult to evaluate in patients with parent vessel occlusion, in whom the aneurysm may be hidden. In such cases, arterial reopening may recanalize the aneurysm, sometimes leading to rupture.14,19 Ideally, when CT or MR imaging depicts an intracranial aneurysm without bleeding, an SAH should be ruled out by CSF spectrophotometric analysis in accordance with published guidelines before a treatment decision.15–17 However, in the context of intravenous thrombolysis, such a procedure is difficult to perform because of the short time window allowed with this treatment and also because it increases the risk of epidural hematoma. The alteplase product license states that an intracranial aneurysm is a contraindication to thrombolysis for acute ischemic stroke even if the data that inform this statement are limited.20 However, guidelines for thrombolytic therapy in acute stroke usually do not retain this condition as an exclusion criterion. Our experience strongly supports a contraindication when an aneurysm may be the source of distal embolization and brain infarct.
The second practical implication when confronted with an aneurysm that embolizes distally is that its status evolves from an asymptomatic to symptomatic state. Because of the risk of subsequent SAH or stroke recurrence within the following days, endovascular or surgical aneurysm exclusion should be considered as early as possible.9,14,21 Medical treatment with aspirin or perhaps with heparin seems to be a reasonable alternative for patients who are not good candidates for endovascular/surgical procedure or while waiting for this treatment.3–6,12 Surgical treatment of aneurysms in the presence of cerebral infarcts seems to be a high-risk procedure, with increased morbidity and mortality.5,6,9 This poorer outcome was significantly demonstrated compared with that of good-grade aneurysms.9 So, whenever possible, endovascular coiling should be offered as the first-line management in these patients and should be performed by experienced teams.
The follow-up of patients with ischemic stroke distal to an aneurysm should be undertaken with caution even if the data suggest that the risk of recurrent ischemic events or rupture after the acute stage is very low, regardless of therapy.3–5 However, considering that even totally thrombosed aneurysms may recanalize, close radiologic follow-up is strongly recommended to detect further reopening,7 and if it occurs, it should be treated adequately and quickly.14 In our experience as in previous studies, none of the patients with ischemic events had additional ischemic episodes after aneurysm treatment.
The relationship between distal embolization from an aneurysmal thrombosis and SAH is unclear. Factors associated with aneurysmal thrombosis are size and, particularly, the ratio of chamber volume to orifice area, blood stagnation, slow flow, and increased blood viscosity.12 Furthermore, turbulent flow within the aneurysmal sac may result in endothelial injury, with exposure of the subendothelial matrix favoring platelet deposition and thrombus formation.11 Brain infarct may result from subsequent distal embolization or parent vessel occlusion due to local extension from the intrasaccular thrombosis. Subendothelial exposure may also induce intramural thrombus and damage of the aneurysmal wall with fragmentation of the elastic lamina and then wall fragility.5,12 Resolution of fresh intramural thrombus associated with the altered wall may lead to rupture or blood extravasation.14
Our study has several limitations owing to its retrospective nature and the small number of patients. It is thus difficult to draw any definite recommendations regarding acute management of aneurysms associated with ischemic stroke, particularly because the occurrence of subsequent SAH has rarely been reported previously. It is possible that we have missed, in our patient selection, those patients with an ischemic stroke distal to an aneurysm not depicted by imaging (for example aneurysms beyond an occluded artery or patients without an MR imaging study). Another concern is whether the aneurysms are the source of embolization or are present incidentally, particularly in those without intrasaccular or arterial thrombosis.4,6 We applied strict selection criteria to reduce the risk of an incidental aneurysm.
Conclusions
Ischemic stroke distal to an unruptured intracranial aneurysm is a rare condition. Our case series indicates that the frequency of early SAH in these patients is high. Therefore, acute management should be undertaken with care regarding antithrombotic use and with asymptomatic meningeal bleeding ruled out by imaging or CSF analysis. To reduce the risk of subsequent bleeding, early endovascular coiling seems a safe and effective treatment and should be considered as the first-line management. Long-term outcome is good for surviving patients. Close radiologic follow-up is required to detect reopening after acute aneurysmal thrombosis.
References
- Received January 22, 2011.
- Accepted after revision March 10, 2011.
- © 2011 by American Journal of Neuroradiology