Abstract
BACKGROUND AND PURPOSE: SHT and ME are feared complications in patients with acute ischemic stroke. They occur >10 times more frequently in tPA-treated versus placebo-treated patients. Our goal was to evaluate the sensitivity and specificity of admission BBBP measurements derived from PCT in predicting the development of SHT and ME in patients with acute ischemic stroke.
MATERIALS AND METHODS: We retrospectively analyzed a dataset consisting of 32 consecutive patients with acute ischemic stroke with appropriate admission and follow-up imaging. We calculated admission BBBP by using delayed-acquisition PCT data and the Patlak model. Collateral flow was assessed on the admission CTA, while recanalization and reperfusion were assessed on the follow-up CTA and PCT, respectively. SHT and ME were defined according to ECASS III criteria. Clinical data were obtained from chart review. In our univariate and forward selection−based multivariate analysis for predictors of SHT and ME, we incorporated both clinical and imaging variables, including age, admission NIHSS score, admission blood glucose level, admission blood pressure, time from symptom onset to scanning, treatment type, admission PCT–defined infarct volume, admission BBBP, collateral flow, recanalization, and reperfusion. Optimal sensitivity and specificity for SHT and ME prediction were calculated by using ROC analysis.
RESULTS: In our sample of 32 patients, 3 developed SHT and 3 developed ME. Of the 3 patients with SHT, 2 received IV tPA, while 1 received IA tPA and treatment with the Merci device; of the 3 patients with ME, 2 received IV tPA, while 1 received IA tPA and treatment with the Merci device. Admission BBBP measurements above the threshold were 100% sensitive and 79% specific in predicting SHT and ME. Furthermore, all patients with SHT and ME—and only those with SHT and ME—had admission BBBP measurements above the threshold, were older than 65 years of age, and received tPA. Admission BBBP, age, and tPA were the independent predictors of SHT and ME in our forward selection−based multivariate analysis. Of these 3 variables, only BBBP measurements and age were known before making the decision of administering tPA and thus are clinically meaningful.
CONCLUSIONS: Admission BBBP, a pretreatment measurement, was 100% sensitive and 79% specific in predicting SHT and ME.
Abbreviations
- ACA
- anterior cerebral artery
- BBBP
- blood-brain barrier permeability
- CBV
- cerebral blood volume
- CTA
- CT angiography
- ECASS
- European Cooperative Acute Stroke Study
- HT
- hemorrhagic transformation
- ICA
- internal carotid artery
- IA
- intra-arterial
- INR
- international normalized ratio
- IV
- intravenous
- MCA
- middle cerebral artery
- ME
- malignant edema
- MMP-9
- matrix metalloproteinase-9
- MTT
- mean transit time
- NCCT
- noncontrast CT
- NIHSS
- National Institutes of Health Stroke Scale
- OR
- odds ratio
- PCA
- posterior cerebral artery
- PCT
- perfusion CT
- PH-2
- parenchymal hematoma type 2
- ROC
- receiver operating characteristic analysis
- SHT
- symptomatic hemorrhagic transformation
- tPA
- tissue plasminogen activator
SHT, the most feared complication in patients with acute ischemic stroke, occurs >10 times more frequently in tPA-treated versus placebo-treated patients.1,2 PH-2—the radiologically defined3 maximally severe form of HT that is often used as an imaging surrogate for SHT in stroke trials4,5—increases the risk of mortality by >10-fold.6 Although the definition and the magnitude of the SHT problem are still under debate,7 there is consensus that minimizing the occurrence of SHT is of paramount importance. The benefits of minimizing SHT go beyond the direct clinical benefits to affected patients. Given that medical and legal concerns about SHT are a primary contributor to the underuse of tPA in potentially eligible candidates despite its indisputable efficacy,8 preventing SHT and assuaging these concerns might increase tPA administration rates for patients within the current tPA administration. Moreover, if a sensitive, specific, and practical method existed to distinguish those at low risk and high risk of SHT, this could increase tPA usage dramatically by facilitating significant extension of the current time window.
In pursuit of a suitable pretreatment method for predicting SHT, numerous clinical and laboratory predictors of PH-2 have been proposed and preliminarily studied.1,9–13 Imaging predictors such as volume of hypoattenuation on admission CT6 and admission diffusion-weighted imaging deficits14 have also been preliminarily studied. These clinical, laboratory, and imaging predictors hold promise, but none have become part of clinical standard of care yet, in part due to concerns regarding sensitivity and specificity. More recently, a promising new approach involving BBBP imaging has been proposed.15–22 It models the extravasation of a contrast agent from the intravascular space to the extravascular space and can demonstrate elevated BBBP as an indicator of ischemia-induced vascular damage in stroke. BBBP imaging provides a physiologic individualized measurement intimately connected to the underlying pathophysiology of PH-2 (ischemia-induced vascular damage followed by reperfusion)23 and may, therefore, offer excellent sensitivity and specificity.
Abnormally elevated BBBP measurements extracted from both PCT15–19 and MR imaging studies20–22 have been explored as physiologic predictors of SHT. Notably, most of the prior PCT studies have used first-pass data,15–18 though a recent study has shown that using first-pass rather than delayed-acquisition data results in inaccurate BBBP measurements.24 One recent PCT study used delayed-acquisition data19; the study involved a quantitative model that had promising predictive power for HT in general but was unable to differentiate between patients destined for PH-2, the only form of hemorrhage definitively linked to poor outcomes in major stroke trials,6 and more minor forms of hemorrhage of the petechial variety. The most recent MR imaging study showed promising predictive power for PH-2 but relied on qualitative analysis by individual experts.22 Our goal was to build on these promising existing studies. Toward this end, we used PCT-based delayed-acquisition data to create a quantitative/automatable prediction algorithm, with the goal of identifying those patients destined for ECASS III-defined SHT2 and PH-2.
In designing our study, we recognized that though SHT and PH-2 have generally received the most attention among investigators, ME is a similarly devastating complication in patients with acute ischemic stroke; indeed, the ECASS III investigators recognized the importance of ME by including it as a primary complication in their study.2
Consequently, we sought to determine whether quantitative PCT-derived BBBP values from a delayed acquisition could predict SHT, PH-2, and ME with high sensitivity and specificity in a consecutive series of patients with acute ischemic stroke. Given the relevance of other clinical and imaging predictors as well, we examined a multivariable model that included admission BBBP values as well as data on reperfusion, recanalization, collateral flow, treatment type (IV tPA, IA tPA, Merci retriever device [Concentric Medical, Mountain View, California], or conservative care), and relevant clinical variables.
Materials and Methods
Study Design
Clinical and imaging data obtained as part of standard clinical stroke care at our institution were retrospectively reviewed with the approval of the institutional review board. At our institution, patients with suspicion of acute stroke and no history of significant renal insufficiency or contrast allergy routinely undergo a stroke CT survey, including NCCT of the brain, PCT at 2 cross-sectional positions, CTA of the cervical and intracranial vessels, and postcontrast cerebral CT, obtained in this chronologic sequence.
We retrospectively identified all consecutive patients admitted to our institution from July 2006 to April 2009 who met the following inclusion criteria: 1) admission to the emergency department with signs and symptoms suggesting acute anterior circulation stroke within 12 hours after symptom onset, 2) documentation of acute ischemic anterior circulation stroke by both admission stroke protocol and clinical examination, 3) no evidence of intracerebral hemorrhage on the admission NCCT, 4) assessment of recanalization and reperfusion via follow-up CTA and PCT protocol obtained between 4 and 30 hours following the admission stroke CT protocol, and 5) assessment of HT via follow-up NCCT following initial treatment.
Clinical Variables and Outcomes
Patients' medical charts were reviewed for demographics, current antiplatelet (aspirin and/or clopidogrel [Plavix]) therapy, current warfarin sodium [Coumadin] therapy, admission INR, admission blood glucose level, admission blood pressure, symptom onset time, time to imaging, time to revascularization therapy, type of revascularization therapy, and NIHSS scores on admission at 24 hours, and on discharge.
Imaging Protocol
PCT studies were obtained on 64-section CT scanners. Each PCT study involved successive gantry rotations performed in cine mode during IV administration of iodinated contrast material. Images were acquired and reconstructed at a temporal sampling rate of 1 image per second for the first 37 seconds and 1 image every 2 seconds for the next 33 seconds. Additional gantry rotations were obtained at 90, 120, 150, 180, 210, and 240 seconds (delayed acquisition). Acquisition parameters were 80 kVp and 100 mAs. Two successive PCT series at 2 different levels (both parallel to the hard palate) were performed to increase brain coverage, with eight 5-mm-thick sections assessed at each PCT level. The first PCT series was obtained at the level of the third ventricle and the basal ganglia. The second PCT series was obtained above the lateral ventricles. For each PCT series, a 40-mL bolus of iohexol (Omnipaque; Amersham Health, Princeton, New Jersey; 300 mg/mL of iodine) was administered into an antecubital vein at an injection rate of 5 mL per second by using a power injector. CT scanning was initiated 7 seconds after the start of the injection of the contrast bolus. The radiation dose was 3.2 mSv. Data from both boluses were used because prior work has demonstrated that there is no significant parenchymal saturation effect from the first bolus and no underestimation of BBBP values from data from the second bolus.25
Image Postprocessing
PCT data were analyzed using commercially available PCT software (Brain Perfusion; Philips Healthcare, Cleveland, Ohio).26 The software applies a closed-form (noniterative) deconvolution to calculate the MTT map, by using previously described methods.27 As stated in prior publications, the deconvolution operation requires a reference arterial input function (most often within the anterior cerebral artery), automatically selected by the PCT software within a region of interest drawn by the user. The CBV map is calculated from the area under the time-attenuation curves compared with a similarly obtained venous reference curve. The PCT infarct core and salvageable brain tissue are automatically calculated by the software by using CBV thresholds and MTT thresholds reported in the literature as the most accurate (PCT salvageable brain tissue: MTT > 145% of the contralateral side values plus CBV ≥ 2.0 mL/100 g; PCT infarct core: MTT > 145% of the contralateral side values plus CBV < 2.0 mL/100 g).28
BBBP measurements were extracted from PCT data by using a second prototype software developed by Philips Healthcare. This software is based on the Patlak model.29,30 Applying the Patlak model to PCT involves the fitting of a regression line to observations of time-attenuation curves for each pixel and for an intravascular reference function. The slope of these regression lines was interpreted as a local blood-to-brain transfer constant and was used as an indicator of BBBP values. BBBP values were presented in color-coded maps generated by the software.
Image Analysis
Matching sections on the admission PCT, on the “reperfusion” PCT, and on the discharge/follow-up NCCT were identified, and only those sections were considered for the image assessment of admission perfusion, reperfusion, and follow-up infarct volume. Of note, because our PCT imaging protocol and the location/orientation of the PCT sections are standardized, the admission PCT sections matched exactly the reperfusion PCT sections in all patients.
Imaging Assessment of Admission Infarct Core and Penumbra
Volumes of infarct core and penumbra, as defined above, on the admission PCT sections were recorded.
Imaging Assessment of Abnormal BBBP
PCT-derived BBBP maps were automatically segmented for all pixels with an absolute BBBP value of >5 mL/100 g/min, which represented the BBBP value superior to the highest BBBP value observed in a series of control patients without an acute ischemic stroke.31 In other words, we defined a threshold for abnormally high absolute BBBP values (>5 mL/100 g/min). Next, we defined a threshold for total volume of pixels with abnormally high (>5 mL/100 g/min) BBBP values. To do so, we tested several thresholds—3, 5, 7, and 9 mL—and found that the 7-mL threshold had maximal sensitivity and specificity in terms of predicting SHT. We then dichotomized patients into 2 groups on the basis of their having a volume of abnormally high absolute BBBP values inferior or superior to the 7-mL volume threshold.
Imaging Assessment of Site and Degree of Occlusion at Admission
On the admission CTA, the degree of occlusion was considered separately in the ICA, supraclinoid ICA, and M1 and M2 segments and was measured from 0% to 100%.
Imaging Analysis of Recanalization
On the “recanalization” CTA, the degree of occlusion was considered separately in the ICA, supraclinoid ICA, and M1 and M2 segments and was measured from 0% to 100%. If an occlusion was seen in 1 of these arterial segments on the admission CTA study, a recanalization index was calculated according to a previously reported method,32 by using the following formula:
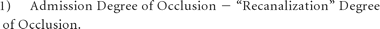
For instance, if the admission CTA showed an occlusion of 100% and if the recanalization CTA showed an occlusion of 50%, the recanalization index was 50%.
Recanalization of the carotid arteries (ICA and supraclinoid ICA) and MCA (M1 and M2) was considered separately. The patients with a carotid recanalization index >50% and an MCA recanalization index >50% were considered to have achieved recanalization; on the other hand, the patients with carotid recanalization index ≤50% or MCA recanalization index ≤50% were considered not to have achieved recanalization. If a 100% recanalization index in 1 arterial segment was accompanied by a negative recanalization index in its distal segment, distal migration of the clot was diagnosed.
Imaging Analysis of Reperfusion
Reperfusion was determined by comparing the admission PCT with the PCT obtained between 4 and 30 hours following the admission stroke CT protocol. Volumes of abnormal MTT (MTT > 145% of the contralateral nonischemic MTT)28 on the admission PCT sections and on the matching reperfusion PCT sections were recorded.
MTT indices of reperfusion, expressed in percentages, were calculated according to a previously reported method32 by using the following formula:

Imaging Analysis of Collaterals
On the admission CTA, the collateral flow was graded according to a previously reported scoring system on a scale from 0 to 333: 0 = absent collaterals, 1 = collaterals filling <50% of the occluded territory, 2 = collaterals filling >50% but <100% of the occluded territory, and 3 = collaterals filling 100% of the occluded territory. The collateral score was then dichotomized into poor (score of 0 or 1) or good (score of 2 or 3).
Determination of SHT and ME
We used ECASS III criteria, which define SHT as any extravasated blood associated with an NIHSS score increase of >4 (or death), in which the HT was the predominant cause of clinical deterioration.2 To make this determination, we reviewed all 24-hour images (and compared them with admission NCCT) for evidence of HT, and we reviewed medical records for documentation of an NIHSS increase >4 that was causally attributed to the HT.
In addition to using ECASS III criteria, we also graded all instances of HT on the basis of a previously reported system by using purely radiologic definitions.3 PH-2, which was 1 of our outcomes of interest, was defined as attenuated hematoma >30% of the infarcted area with a substantial space-occupying effect or as any hemorrhagic lesion outside the infarcted area.
Follow-up imaging was also reviewed for ME as a primary complication, as was done in ECASS III.2 ME was defined as brain edema with mass effect as the predominant cause of clinical deterioration (an NIHSS score increase of >4 or death).
Statistical Analysis
Our outcome was significant clinical deterioration (an NIHSS score increase of >4) related to either SHT or ME. We conducted a univariate analysis to evaluate the predictive value of proposed clinical and imaging predictors. In terms of clinical variables, we included age, current antiplatelet (aspirin and/or clopidogrel) therapy, current warfarin therapy, admission INR, admission blood glucose level, admission systolic blood pressure, admission NIHSS score, time from symptom onset to scanning, Merci treatment, and tPA treatment. In terms of imaging variables, we included admission volume of abnormally high BBBP, volume of admission PCT-defined infarct, collateral score, recanalization, and reperfusion. For each variable, we also performed an ROC, and we recorded the area under the curve values as an indicator of the accuracy of each proposed predictor.
Subsequently, a multivariate mixed-effect model involving forward-stepwise selection with a significant threshold set at .05 was built from the variables that had a univariate P value < .2. The area under the curve of the final multivariate model was calculated.
Results
Patient Characteristics
We identified 32 patients admitted to our institution between July 2006 to April 2009 who met the inclusion criteria. Patient characteristics, as well as imaging assessment of admission infarct core, penumbra, and reperfusion, are summarized in Table 1.
Study patient characteristics (N = 32)
Imaging Assessment of Admission Site and Degree of Occlusion, Recanalization, and Collaterals
On the admission CTA study, 29 of 32 patients had an acute arterial occlusive lesion. One patient had only carotid occlusive lesions (ICA and supraclinoid ICA), 24 had only MCA occlusive lesions (M1, M2, or both), and 4 had both carotid and MCA occlusive lesions. The ICA was completely occluded in 4 patients, the supraclinoid ICA was completely occluded in 4 patients, the M1 segment was completely occluded in 20 patients and 50% occluded in 1 patient, and the M2 segment was completely occluded in 14 patients and nearly completely occluded in 3 patients (90%, 95%, and 95% occluded). In the 3 remaining patients, no arterial occlusive lesion could be detected on the admission CTA study, even though PCT findings showed territorial perfusion deficits compatible with acute ischemic stroke.
In the patient who had only carotid occlusive lesions, recanalization was not achieved. In the 24 patients who had only MCA occlusive lesions, recanalization was achieved in 15. Of the 4 patients who had both carotid and MCA occlusive lesions, recanalization was achieved in 1.
Distal migration of the clot was diagnosed in 5 patients. Three had complete M1 segment occlusion on the admission CTA study followed by occlusion of ≥1 M2 segment branch at the recanalization CTA study. Two had complete occlusion of an M2 segment on the admission CTA study followed by occlusion of ≥1 M3 segment branch at the recanalization CTA study.
On the admission CTA study, 9 patients had poor collateral flow (score of 0 in 3 patients, score of 1 in 6 patients) and 23 patients had good collateral flow (score of 2 in 11 patients, score of 3 in 12 patients).
Clinical and Imaging Outcomes
Eight patients showed a significant clinical deterioration (an NIHSS score increase of >4 or death). Three of these 8 had SHT (of these 3, 2 received IV tPA, while 1 received IA tPA and treatment with the Merci device). These same patients all had PH-2 and were the only patients who had PH-2, indicating perfect overlap in our study between the ECASS III criteria for SHT and the radiologic criteria for PH-2, in agreement with previous studies.6 Another 3 patients had ME (2 received IV tPA, while 1 received IA tPA and treatment with the Merci device). Finally, there were 2 patients in our study who had significant clinical deterioration due to non-neurologic causes—aspiration pneumonia and septicemia—without SHT or ME.
These 6 patients with a significant clinical deterioration (an NIHSS score increase of >4 or death) and with either SHT3 or ME3 were considered as those with positive outcomes.
Univariate Analysis of Clinical and Imaging Predictors of Significant Clinical Deterioration
Variables associated with a P value < .2 in the univariate analysis (Table 2) in terms of predicting a significant clinical deterioration due either to SHT or ME were age, admission NIHSS, tPA treatment, admission PCT−defined infarct volume, admission volume of abnormally high BBBP, and reperfusion score. Nonsignificant variables in the univariate analysis were current warfarin therapy, admission INR, admission blood glucose level, admission systolic blood pressure, time from symptom onset to scanning, Merci treatment, and recanalization.
Univariate analysis of clinical and imaging predictors of significant clinical deterioration
Reperfusion scores in patients with SHT and ME were significantly lower (P = .003) than those in patients without complications. We determined that this difference was likely the consequence of the timing of the reperfusion imaging studies. All the reperfusion studies in the patients with SHT were obtained after the PH-2 had already developed, so the PH-2 complication itself was the cause, rather than the consequence, of the low reperfusion score. Consequently, we did not include reperfusion in our forward selection−based multivariate analysis.
Use of aspirin and/or clopidogrel was absent (P = .007) in all of patients with SHT and ME. The directionality of this significant difference was the opposite of what we would expect, given that aspirin and clopidogrel have antiplatelet effects with a relatively long duration of action. Consequently, we did not include aspirin and/or clopidogrel usage in our forward selection−based multivariate analysis.
Forward Selection−Based Multivariate Analysis
The multivariate analyses demonstrated that the optimal model to predict significant clinical deterioration related to either SHT or ME included age, tPA administration, and admission volume of abnormally high absolute BBBP values (Table 3, Fig 1). The optimal threshold to dichotomize age was 65 years. The optimal threshold to dichotomize the volume of abnormally high absolute BBBP values was 7 mL.
Forward selection−based multivariate analysis and area under the curve optimization in patients with acute ischemic stroke
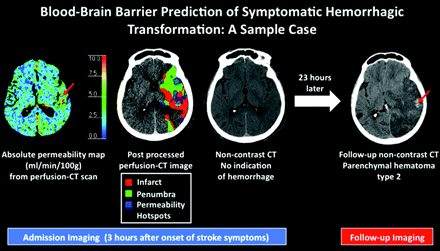
A 75-year-old woman was admitted to the emergency department with a right hemiparesis. She underwent stroke CT imaging work-up approximately 2 hours after symptom onset. NCCT revealed no evidence of intracranial hemorrhage, and she was treated with IV tPA at this time. Twenty-three hours later, she was in critical condition in the intensive care unit, and imaging follow-up at that time demonstrated development of PH-2. There are 2 red arrows—1 on the admission permeability map and 1 on the follow-up NCCT. The red arrows indicate that the patient presented with a “hotspot” (a sizable volume of abnormally elevated permeability on the baseline PCT study); this hotspot was centered in the same place as a focus of significant hemorrhage in the patient's eventual PH-2. Of note, permeability hotspots (voxels with absolute permeability >5 mL/100 g/min that are delineated in blue automatically by the software) occur in both the infarct and penumbra, not just in the infarct, where the vasculature has presumably undergone the most severe ischemia-induced damage. Thus, permeability imaging provides information above and beyond what is provided by the standard PCT parameters that can be used to define infarct and penumbra.
Prediction of SHT/PH-2 or ME
The sensitivity and specificity of admission BBBP measurements as the exclusive predictor of SHT and ME were 100% and 79%, respectively (On-line Fig 1). In this analysis, there were 5 false-positives (patients with admission BBBP above the threshold who did not subsequently develop SHT or ME). Median admission volumes of abnormally high absolute BBBP values were 8.9 (range, 7–17) in those patients who developed SHT or ME. Median admission volumes of abnormally high absolute BBBP values were 1.4 (range, 0–42) in those patients who did not develop SHT or ME (On-line Table).
The sensitivity and specificity of admission BBBP above the threshold and age ≥65 years were 100% and 81%, respectively. In this analysis, there were 3 false-positives (patients with both admission BBBP above the threshold and age ≥65 years who did not subsequently develop SHT or ME). None of these 3 patients received tPA (2 received conservative care and 1 received treatment with the Merci device).
The sensitivity and specificity of admission BBBP above the threshold and tPA administration together were 100% and 88%, respectively. In this analysis, there were 2 false-positives (patients with admission BBBP above the threshold who received tPA but who did not subsequently develop SHT or ME). These 2 patients were only 39 and 49 years of age, whereas the mean age among the 6 patients with SHT and ME was 81 years, and the youngest in this group was 65 years of age.
The sensitivity and specificity of admission BBBP above the threshold, age ≥65, and tPA administration together (a triad of characteristics) were 100% and 100%, respectively.
Discussion
This study highlights the promise of using quantitative BBBP imaging in an automatable algorithm to predict subsequent development of SHT or ME in patients with acute ischemic stroke. Using admission BBBP above a volume threshold as the sole predictor already yields a sensitivity of 100% and specificity of 79% (5 false-positives); given that this is a pretreatment measurement, it suggests a significant potential role for BBBP imaging in the clinical context. This finding is in agreement with the critical role of ischemia-induced vascular damage in the pathogenesis of SHT and ME that has been previously reported.23,34 If we add age ≥65 years to our model, specificity becomes 81%; and if we use the triad of admission BBBP above the threshold, age ≥65 years, and tPA administration, we can predict subsequent development of SHT or ME with 100% sensitivity and 100% specificity because 2 of 5 of the false-positives based on using BBBP imaging alone were younger than 65 years and the remaining 3 false-positives based on using BBBP imaging alone did not receive tPA. Of these 3 variables, only BBBP measurements and age are known before making the decision of administering tPA and thus are clinically meaningful.
The role of age ≥65 years and tPA administration in maximizing the specificity of our algorithm is consistent with prior studies that have discussed the relevance of these 2 variables in the development of SHT. Older age in tPA-treated patients was associated with SHT in an analysis of National Institute of Neuroloical Disorders and Stroke trial data.7 In addition, a secondary analysis of the ECASS II trial demonstrated that age was an independent predictor of SHT12; on a related note, the investigators in ECASS III went so far as to include only patients 80 years of age and younger in their study,2 in part because of the putative association between older age and SHT. While the link between older age and ME is not documented like the link between age and SHT, our study suggests such a link, which is physiologically plausible given that the pathogenesis of SHT and ME share certain similarities regarding vascular damage.23,34
The role of tPA in SHT pathogenesis has been unequivocally established and widely studied. Although tPA offers an indisputable net overall benefit in patients with stroke,2 all major clinical trials of tPA have demonstrated its role in significantly increasing the risk of SHT.1,2,6,35 Furthermore, studies have suggested that tPA not only increases the risk of SHT by promoting reperfusion but also by direct upregulation of MMP-9,36–38 a protease that damages the vasculature. tPA upregulation of MMP-9 likely also plays a similar role in the pathogenesis of ME, given that elevated MMP-9 levels have been demonstrated in patients with stroke who develop ME.39
Most interesting, our data on collaterals, which were derived from admission CTA imaging, added additional information and provided a way to potentially distinguish patients with SHT and ME. Although we were not able to conduct a formal statistical analysis secondary to the small number of patients with SHT and ME and, therefore, do not have statistically significant results, we noted that all the patients with SHT had good collateral scores, whereas all the patients with ME had poor collateral scores. This is a new observation, albeit 1 that is limited in significance due to the small sample size and lack of statistical significance. To explain this finding, we need to recognize that reperfusion has been clearly implicated in the pathogenesis of SHT23 but not of ME; furthermore, reperfusion can occur not only via successful recanalization of the primary occlusion and restoration of downstream flow but also via good collaterals.40 Therefore, the link between SHT and good collaterals makes sense in terms of our current understanding of the pathophysiology of SHT and ME.
In contrast to our findings regarding collaterals, our findings regarding reperfusion are, on initial inspection, quite counterintuitive. Reperfusion was a statistically significant predictor of SHT in the univariate analysis, but the reperfusion scores in patients with SHT were lower than those in other patients, not higher as we would have expected on the basis of the role of reperfusion in SHT.23 This apparent contradiction can be resolved by recognizing that our study was retrospectively conducted with follow-up imaging performed for clinical purposes rather than follow-up imaging optimized for research purposes. The reperfusion scans in the patients with SHT were conducted at the time that PH-2 had already developed, so the PH-2 complication itself severely impacted the reperfusion score. PH-2 likely had a compressive effect on the vasculature that resulted in the poor reperfusion scores. Ideally, in a prospective study with optimized time points for imaging follow-up,41 we would have obtained follow-up imaging at 2 separate time points—a dedicated reperfusion scan soon after treatment (but before the development of PH-2 or ME) and a later follow-up scan to assess the development of PH-2 or ME. In this ideal situation, what we likely would have seen in our patients with SHT is good reperfusion on the reperfusion scan but artifactual poor reperfusion on the later follow-up scan after PH-2 had already developed.
In general, our study is limited due to the fact that it was retrospective and conducted at only 1 institution. Moreover, 1 significant limitation of our study is our small sample size of only 32 patients and only 3 patients with SHT and 3 patients with ME. In a sense, however, the fact that our sample size was relatively small and yet we still demonstrated promising sensitivity/specificity and statistically significant results in the multivariate analysis points to the promise of PCT-based BBBP imaging and suggests that a prospective multicenter trial is indicated. Furthermore, our decision to combine patients with SHT and ME as a single outcome is reasonable given that both SHT and ME are devastating complications with ischemia-induced vascular damage as a central component of their pathogenesis.23,34 Our objective was to demonstrate that BBBP imaging can predict both of these clinically relevant complications.
One general limitation of using our method of BBBP imaging is that it depends on the arrival of a contrast agent. There may be instances where blood flow and contrast arrival are nonexistent, in which case permeability measurements would not be possible until reperfusion is achieved. In these instances, if we were to conduct an admission scanning in a patient with a 100% occlusion and no collateral flow, we would reach a conclusion of indeterminate permeability because it could be low or high. If we were to proceed cautiously and withhold tPA in the face of indeterminate permeability, we would be incorrectly doing so if the permeability was indeed low. On the other hand, if we were to proceed aggressively in this scenario and administer tPA in the face of indeterminate permeability, we would be incorrectly doing so if the permeability was indeed high. While this is certainly a limitation of our methods, the incidence of such a “no flow, no contrast” phenomenon (complete occlusion and no collateral flow) is quite low. In the patient population in our study, we found that the incidence of both complete occlusion and no collateral flow was <10%; in other words, there was usually at least some minimal degree of flow. In such instances where there is some minimal degree of flow, the highly sensitive algorithm that we use can calculate permeability, which is why we reliably observed increased permeability even in infarcted areas with very low CBF. For our algorithm, it does not matter whether the blood flow is antegrade or retrograde—it only matters that some contrast arrives. Ultimately, given the rarity of this phenomenon of “no flow, no contrast,” using BBBP imaging to individualize the risk assessment for SHT or ME might still be superior to using a generalized time window.
With regard to the clinical utility of BBBP imaging, we would like to emphasize that the ultimate goal of using BBBP imaging for predicting and preventing complications is to extend the time window and increase tPA administration rates, not diminish them. Extending the time window for tPA administration on an individualized basis requires identifying patients for whom there is a large potential benefit of reperfusion therapy due to substantial remaining penumbra and a small potential risk of reperfusion therapy due to a mostly intact vasculature (in other words, a low risk of SHT). While tPA might be selectively withheld from those patients deemed at greatest risk of SHT, the remaining patients at low risk of SHT—who would constitute most, given that SHT rates are still only approximately 10%, even with imaging-selected patients at ≤9 hours42—could be given the “green light” for tPA, assuming that there was also substantial remaining penumbra. In this manner, treatment could be individually optimized to minimize the incidence of SHT while maximizing the number of patients treated.
Specifically, we show that PCT-based BBBP imaging is quantitative and automatable. As a means to assess the potential risk of thrombolytic therapy, this form of BBBP imaging complements existing quantitative automated methods28 that delineate the penumbra and infarct and assess the potential benefit of reperfusion. Ultimately, larger studies will need to be conducted prospectively at multiple centers to validate our results, our BBBP thresholds, and our predictive model of SHT and ME.
Footnotes
-
Indicates Editor's Choices selection
-

-
Max Wintermark receives funding from the National Center for Research Resources, grant KL2 RR024130; GE Healthcare; and Philips Healthcare. He is a consultant for Concentric. Jason Hom receives funding from the National Institutes of Health National Center for Research Resources, UCSF-CTSI grant UL1 RR024131. Joerg Bredno is an employee of Philips Healthcare.
-
The content of the article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurologic Disorders and Stroke, National Center for Research Resources, National Institutes of Health, or the other sponsors.
-
Indicates article with supplemental on-line tables.
-
Indicates article with supplemental on-line figures.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received January 5, 2010.
- Accepted after revision June 9, 2010.
- Copyright © American Society of Neuroradiology