Abstract
SUMMARY: MR imaging of peripheral nerves has been described in relation to abnormalities such as nerve injury, entrapment, and neoplasm. Neuroma formation is a known response to peripheral nerve injury, and here we correlate the MRN appearance of postinjury neuroma formation with intraoperative findings. We also present the MR imaging features of surgical treatment with a synthetic nerve tube and nerve wrap on postoperative follow-up imaging.
Abbreviations
- MRN
- MR neurography
- NIC
- neuroma in continuity
- SPACE
- sampling perfection with application optimized contrasts by using different flip angle evolutions
- SPAIR
- spectral adiabatic inversion recovery
- STIR
- short tau inversion recovery
Peripheral nerve injuries and entrapments may lead to formation of NIC, neuroma in completely severed nerves, and amputation neuroma. These lesions also demonstrate unique MRN appearances. This article presents MRN and surgical correlations of various posttraumatic neuromas with relevant case examples.
Anatomy and Pathophysiology
The basic structure of a peripheral nerve is a neuronal axon enveloped in a myelin sheath composed of Schwann cells and loose connective tissue referred to as the “endoneurium.” Multiple axons are arranged together as bundles called “fascicles,” the fundamental neural unit imaged with current high-resolution MRN techniques (Fig 1). Each fascicle is enveloped by a connective tissue layer called the “perineurium.” Groups of fascicles are arranged in yet another connective tissue layer, called the “epineurium,” which serves as the outer sheath of the peripheral nerve.1,2 In traumatic or entrapment neuropathies, the nerves can go through a spectrum of injuries spanning neurapraxia (focal damage to the myelin sheath without axonal disruption), axonotmesis (disruption of multiple axonal fibers without myelin or connective tissue disruption), and neurotmesis (complete loss of axonal continuity along with partial or complete discontinuity of the myelin sheath and supporting connective tissue fibers).
3D STIR SPACE in an oblique coronal reconstruction (A) and axial T2 SPAIR (B) images demonstrate normal fascicular appearance of the sciatic nerves (arrows).
A neurapraxia by definition resolves very quickly (within weeks to a few months) because the axons are not damaged and, therefore, do not undergo wallerian degeneration, the process of axonal degradation distal to a site of nerve injury. These injuries are treated conservatively and will fully recover. However, injuries associated with an axonotmesis or neurotmesis do experience wallerian degeneration; therefore, the nerve has to regenerate from the point of injury to its distal target. The advantage for the patient with an axonotmesis is that the neural track and Schwann cell tract are still intact so that regeneration to the distal targets is more accurate and, therefore, will usually recover good function if treated conservatively. In neurotmesis, little recovery can be expected without surgical intervention because the nerve is physically divided and the orientation of the nerve is disrupted. Surgical exploration is performed to reorient the nerve fiber and repair the nerve.3–5
Pathologically, any nerve that is lacerated, avulsed, or traumatized may form a neuroma. These neuromas can be classified into 2 basic types: NIC or an end-bulb neuroma. NIC usually involves all degrees of nerve injury, from normal to neurotmesis, coexisting within a scarred nerve. With time, the proximal injured nerve fascicles sprout in an attempt to unite. However, due to surrounding lattice disruption, disorganized regeneration, hypertrophy of nerve fascicles, and associated fibrosis, the proximal and distal nerve fibers at the site of injury may fail to appose and thereby lead to NIC formation. NICs may be of 2 types pathologically: spindle neuromas with intact perineurium or lateral neuromas that occur after partial disruption of the perineurium and after nerve repairs.6–8
End-bulb neuromas occur anywhere a nerve is completely divided and is unopposed by another neural tissue. These are subcategorized as a neuroma in a completely severed nerve and as an amputation neuroma. Most commonly these occur in lacerations in which the nerve is not repaired in a timely fashion, amputation stumps, and in postoperative patients in whom sensory nerves in the skin may have been divided unknowingly.
Clinical Presentation and Management
Clinically, NIC may lead to a dysfunctional nerve, disabling pain, alterations in the patient's lifestyle, and possible progression to chronic pain syndromes. The Tinel sign (local tenderness over the injured nerve with distally radiating tingling), denervation atrophy of the muscles, and sensory or trophic changes are evident on clinical examination. Treatment of NIC is challenging and is targeted to alleviating the pain and restoring the functional loss caused by the nerve injury.7
Recent microneurosurgical techniques are a considerable advancement in repairing these lesions. After failure of conservative treatment, if the patient continues to have severe pain or loss of sensory or motor function, nerve exploration is performed. On exploration, an aggressive neurolysis is performed to free the nerve from adhesions and to remove scar tissue in and around the nerve itself. If the patient has a total loss of function and exploration reveals a very badly injured nerve and no function can be restored with intraoperative nerve stimulation, nerve resection and reconstruction may be required. However, if the patient has partial function of the affected nerve, often a neurolysis will be sufficient to allow regeneration. Surgeons have tried for decades to prevent recurrent scarring around the released nerve to help prevent traction neuritis. There is controversy over how best to do this. Some prefer to start active and passive range of motion early to allow the nerve to “glide,” preventing adhesions. Others have advocated wrapping the nerve with substances designed to limit scarring. These substances have ranged from silicone tubes, umbilical veins, autologous veins, and now highly engineered collagen-based nerve tubes and nerve wrap (Fig 2D). The collagen wrap generally resorbs in a few months' time, depending on the construct.9
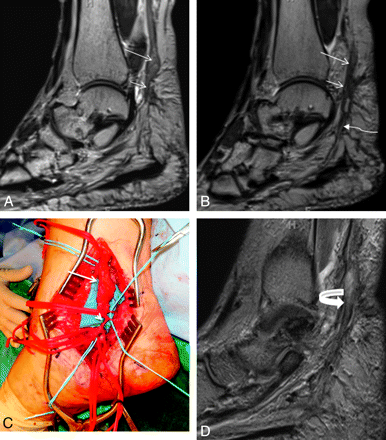
A 46-year-old woman with a history of attempted right tarsal tunnel release presented with persistent foot and ankle pain with numbness in the medial plantar distribution. A and B, Sequential sagittal T2 SPACE images demonstrate a spindle-shaped NIC involving the distal tibial nerve (long arrows). Notice the attenuated appearance of the medial (short arrows) and lateral (wavy arrow) plantar nerves. C, Intraoperative photo confirmed the NIC (long arrow) and small proximal medial and lateral plantar nerves entrapped in scarring (short arrow). D, Extensive neurolysis was performed and a nerve wrap was placed. Follow-up sagittal T2 SPACE MR image shows the wrap as a hypointense covering around the tibial and proximal medial plantar nerve (curved arrow), with residual hyperintensity of the nerves.
A 48-year-old woman with a history of attempted left tarsal tunnel release and midfoot fusion presented with severe pain in the bottom of the foot with toe flexion weakness. A and C, Sagittal T2 SPACE (A) and axial T2 SPAIR images (C) demonstrate a spindle-shaped NIC involving the tibial nerve (arrow), medial plantar nerve (short arrow), and relatively less involved lateral plantar nerve (wavy arrow) entrapped in surrounding scarring. Extensive denervation edema and atrophy of plantar muscles were also identified (not shown). B, The findings correlate well with intraoperative photography. D, Collagen-based nerve wraps are placed around the neurolysed segments of medial and lateral plantar nerves (curved arrows), with successful recovery.
As mentioned, in all end-bulb neuromas or in severe cases with complete loss of function associated with a NIC, the neuromatous area is completely excised and the nerve is reconstructed. There are a number of ways to reconstruct nerve injuries. The traditional method is usually with nerve grafting if the 2 cut ends of the nerve cannot come together without tension. However, other options now exist. A variety of nerve conduits now allow the surgeon to suture the conduit to the proximal and distal end of the injured nerve instead of harvesting a nerve graft. Such conduits allow the proximal axons to regenerate distally in a protected environment to reach the distal stump and have been shown to be as effective or more so compared with nerve grafts for nerve defects up to 3 cm in length.10–15
Neuromatous noncritical sensory nerves are often successfully treated with excision of the neuroma and implantation of the proximal cut end into a muscle to help prevent recurrent neuroma formation.16
MRN
MRN imaging of peripheral nerves depends in part on fat-suppressed fluid-sensitive sequences for detection of nerve abnormalities. Together with T1-weighted imaging, these techniques may be used to demonstrate changes in nerve size, signal intensity, and course. Current high-field systems, used with evolving methods of fat suppression, promise to advance the diagnostic capability of MRN.
Peripheral nerve MR imaging is performed at our institution by using 3T imaging systems (Magnetom Trio, Magnetom Verio; Siemens, Erlangen, Germany) in combination with thin-section planar (ie, 2- to 3-mm section thickness) and isotropic 3D SPACE sequences (eg voxel dimensions from 0.8 mm to 1.0 mm). 3D acquisition with isotropic voxel resolution allows arbitrary multiplanar postprocessing, such as the oblique coronal plane used to demonstrate the sciatic and tibial nerves (Figs 1A, 2, and 3). Fat suppression is achieved with STIR (Fig 1A) and T2 SPAIR techniques, the latter providing improved insensitivity to field heterogeneity compared with traditional chemical fat suppression while also allowing a better signal intensity–to-noise ratio compared with STIR sequences (Fig 1B). Isotropic 3D pre- and postgadolinium T1-weighted imaging is also selectively performed, by using volumetric interpolated breath-hold sequences.
In neurapraxia and axonotmesis, MRN usually demonstrates enlarged and T2 hyperintense nerves due to various hypothesized mechanisms, such as, proximally, due to obstructed axoplasmic flow (Fig 4) and, distally, due to wallerian degeneration. The increasing abnormal T2 hyperintensity of the nerve fascicles correlates with the severity of the nerve injury. Also, a return to normal size and signal intensity within the nerve correlates with functional recovery. Distal denervation muscle atrophy serves as a useful secondary sign of nerve injury on MR imaging.17–19
A 42-old-woman with tingling and numbness in the ulnar side of the right hand. Axial T1 (A) and T2 SPAIR (B) images show a hyperintense ulnar nerve (long arrows) entrapped at the cubital tunnel due to focal fibrosis (short arrows) related to previous injury. No denervation atrophy was seen at the time of imaging, and clinical as well as electromyography findings were in keeping with neurapraxia.
MRN can also depict true discontinuity in the nerve in cases of neurotmesis, though hemorrhage in acute stages can obscure the findings. MRN demonstrates the NIC as a baseball-shaped mass with nerve continuity on either side (Figs 2 and 3). To our knowledge, NIC has not been described previously in the radiology literature. The MRN appearance of NIC is somewhat similar to that of neurogenic tumors such as schwannomas and neurofibromas. However, in our experience, NIC may often be distinguished from neurogenic tumors (Fig 5) by the presence of surrounding scarring, lack of a split fat or target sign, and absence of abnormal enhancement. A neuroma in a completely severed nerve (end-bulb neuroma) demonstrates a T2 hyperintense nerve terminating in a baseball-shaped mass resembling a balloon on a string or a green onion appearance (MRN not shown; see surgical photograph, Fig 6D). Amputation neuroma (ie, end-bulb neuroma), as the name suggests, arises in an amputated limb and otherwise demonstrates an appearance similar to neuroma in a completely severed nerve (Fig 7).
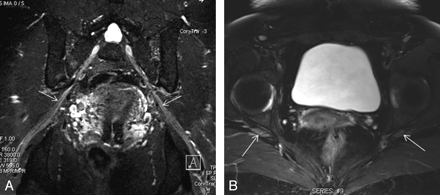
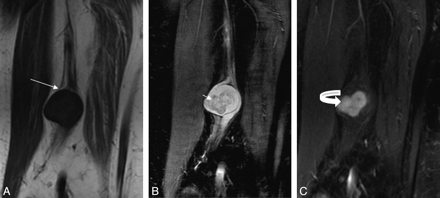
Benign peripheral nerve sheath tumor of the sciatic nerve shows the typical split fat sign (arrow) on coronal T1 (A), target sign (short arrow) on coronal STIR (B), and nodular enhancement (curved arrow) on a postcontrast coronal T1 fat-saturated image (C).
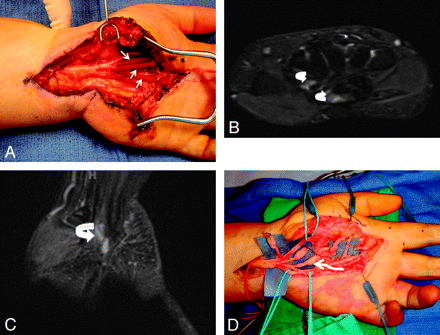
A 49-year-old woman with a history of previous carpal tunnel surgery and median nerve injury. A, The median nerve was re-explored in the hand followed by neurolysis and nerve tube placement (short arrows). The patient did not recover nerve function following surgery. B and C, MR imaging examination 5 months after surgery shows no significant nerve regeneration and empty fluid-filled nerve tubes (curved arrows). D, Re-exploration demonstrated end-bulb neuromas (wavy arrow), and nerve grafting was performed.
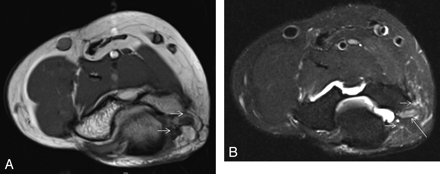
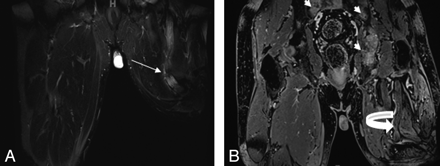
A 50-year-old man with previous left leg amputation for epitheloid sarcoma presents with multiple bony metastases. A and B, Notice an enlarged nonenhancing hyperintense sciatic nerve with an amputation end-bulb neuroma on coronal STIR (arrow, A) and postcontrast T1 3D gradient recalled-echo (curved arrow, B) images.
In cases of surgical neuroma resection, peripheral nerve reconstruction may be performed with either nerve grafts or nerve conduits, as described above, and conduits have been found to allow native axonal regeneration. We have found nerve wraps and conduits to exhibit curvilinear T1 and T2 signal-intensity hypointensity on postsurgical follow-up MRN, as shown in Figs 2D, 6, and 8.
MRN techniques may also be helpful in postsurgical follow-up after nerve reconstruction. In the setting of peripheral nerve reconstruction with synthetic conduits, postoperative follow-up MRN has the potential to demonstrate nerve regeneration. Small areas of nonenhancing soft-tissue intensities within the conduit (Fig 8) are hypothesized to represent early regenerating nerve sprouts. However, a controlled study is warranted to further evaluate the postoperative MRN appearance of nerve regeneration and to correlate these findings with clinical and electrophysiologic studies. A better understanding of the postoperative appearance of nerve regeneration through conduits could impact management in cases of failed response. Nerve transfers may represent a salvage treatment option in such cases (Fig 6D).
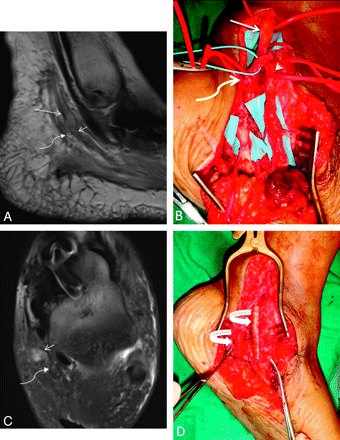
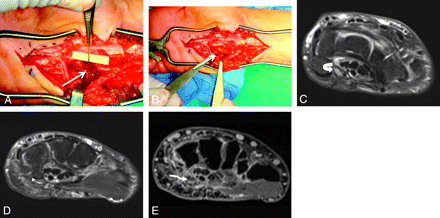
A 48-year-old woman presented with a claw hand following injury to the ulnar nerve during ganglion cyst removal from Guyon canal. A and B, During repeat surgery, the severed nerve is sutured to the ends of a neurotube (arrows). C−E, MRN examination was performed 1 month after the surgery. Axial T2 SPAIR image (C) shows an enlarged and hyperintense ulnar nerve proximally related to long-standing obstruction of axoplasmic flow (curved arrow) and postoperative changes. At the level of the hook of the hamate, minimal filling of the neural tube with nonenhancing tissue (arrowhead on the axial T2 SPAIR image in D, and wavy arrow in the postcontrast 3D T1 fat-saturated image in E) is hypothesized to represent early nerve sprouts.
Conclusions
Peripheral nerve MRN is a useful technique for the preoperative diagnosis, localization, and characterization of nerve abnormalities, including posttraumatic neuroma formation. In addition, these techniques promise to play a role in postsurgical evaluation after nerve reconstruction. Radiologists should be aware of the MRN appearance of injury-related neuromas for appropriate diagnosis and avoid misinterpretation as true neoplasms.
Footnotes
-
K.C.W. gratefully acknowledges the support of the Radiological Society of North America (RSNA) Research and Education Foundation Fellowship Training grant #FT0904, as well as that of the Walter and Mary Ciceric Research Award.
Indicates open access to non-subscribers at www.ajnr.org
References
- Copyright © American Society of Neuroradiology