Abstract
SUMMARY: MR imaging is widely used for the diagnosis and monitoring of patients with MS. Applications and protocols for MR imaging continue to evolve, prompting a need for continual reassessments of the optimal use of this technique in clinical practice. This article provides updated recommendations on the use of MR imaging in MS, based on a review of the trial evidence and personal experiences shared at a recent expert meeting of radiologists and neurologists.
Abbreviations
- BBB
- blood-brain barrier
- CIS
- clinically isolated syndrome
- CNS
- central nervous system
- DTI
- diffusion tensor imaging
- DWI
- diffusion-weighting imaging
- FLAIR
- fluid-attenuated inversion recovery
- Gd
- gadolinium
- IFNB
- interferon β
- MS
- multiple sclerosis
- NSF
- nephrogenic systemic fibrosis
MR imaging has played an increasing role in the diagnosis and management of MS.1 It offers 3 main applications in MS. First, in combination with characteristic symptoms, it provides earlier and more confident diagnosis than symptoms alone.2 Second, it contributes to our understanding of the pathophysiology of MS and how pathophysiologic changes relate to clinical manifestations of disease.3 Third, it has a role in monitoring the effects of therapies in clinical trials and also has the potential to identify response to therapy in individual patients.4,5
Contrast agents are conventionally included in acquisition protocols for MR imaging. Use of these agents also offers insights into pathogenesis and enhances the monitoring of treatment effects.6
As applications for MR imaging in MS evolve and increasing numbers of techniques and protocols are adopted, there is a trend toward variation in the use of MR imaging among centers. In the face of this growing divergence, experts recognize the importance of standardizing protocols based on evidence of optimal practice.6 This review seeks to contribute to this important objective by reporting an expert meeting that focused on discussions and recommendations for the optimal use of MR imaging in MS by neurologists and radiologists—key participants in the use of contrast-enhanced MR imaging in MS.
Applications of MR Imaging in MS
MR imaging offers clinicians a range of applications for the management of MS, including support in diagnosis, insights into pathogenesis, an understanding of prognosis relevant to individual patients, and assistance in monitoring the effects of therapy.
Support in Diagnosis
A diagnosis of MS is founded on clinical evaluation. Several criteria have been developed to integrate MR imaging with clinical evaluation and other diagnostic methods to achieve earlier and more accurate diagnosis, including the revised McDonald criteria.2
The McDonald criteria were the first to incorporate the brain and spinal cord lesions visualized on MR imaging into traditional diagnostic approaches, including history, examination, and laboratory tests.7 The revised McDonald criteria included amendments to the original guidelines to facilitate use in typical practice settings.2 Specifically, these revisions were designed to help demonstrate lesion dissemination in time, clarify the evaluation of spinal cord lesions, and simplify the diagnosis of primary-progressive disease.
The revised McDonald criteria have largely superseded earlier criteria7–9 and represent an important element in the diagnosis of patients with suspected MS. The revised McDonald criteria have, however, been criticized for their perceived complexity and low (∼60%) sensitivity.10
Less complex criteria have been produced, such as the Swanton criteria, which are claimed to offer similar specificity (87%) but increased sensitivity (72%) compared with the McDonald criteria.10 Despite apparent advantages for the Swanton criteria, there has been hesitation among most clinicians to adopt them.
Another study investigating simplified criteria for diagnosing MS reported that a single MR imaging study performed <3 months after the onset of CIS is highly specific for the development of clinically definite MS in the presence of dissemination in space, providing that both gadolinium-enhancing and -nonenhancing lesions are found, indicative of dissemination in time.11 These interesting results require confirmation.
Consensus Statement.
MR imaging has an important role in the diagnosis of MS. Expert meeting participants recommended adopting standardized protocols and reporting procedures based on the revised McDonald criteria.
Regional Differences in Characteristics of MS Lesions
The presentation of MS typically differs between Asian and Western populations.12 In Asian patients, the optic-spinal type of MS is more frequent, an older age group (>35 years) is affected, fewer cases are positive for oligoclonal bands, and total protein concentrations in CSF are higher. When spinal involvement occurs, the lesion is characteristically longer. Caution should be exercised when applying the McDonald criteria in Asian patients because of the characteristic differences in lesion location compared with Western patients. Inclusion of a spinal cord lesion as a juxtacortical lesion increases sensitivity for diagnosis.13 The criteria of Poser et al9 are considered more reliable than the McDonald criteria in Asian populations, though they are not dependable for early diagnosis.
Consensus Statement.
MR imaging evaluation in Asian populations should focus on the optic nerve and spinal cord. Given the frequency of spinal cord involvement in Asian patients with MS, McDonald criteria require modification for these patients.
Insights into Pathogenesis
MS is a complex immune disease in which self-reactive T-cells and monocytes mediate inflammation of CNS white matter and demyelination of axons, leading typically to cumulative neurologic disability.14 MR imaging provides insights into the pathogenic processes of MS, alongside other noninvasive techniques and clinical evaluation. In particular, MR imaging by using gadolinium-containing contrast agents has helped identify the pivotal role of the BBB.15 Breaching the BBB by immune cells mediates structural and functional changes in the CNS of patients with MS, including inflammation, demyelination, axonal loss, remyelination, and gliosis. Demonstrating BBB disruption at MR imaging may represent one of the earliest indications for a diagnosis of MS. Insights into pathogenesis provided by MR imaging have also offered greater understanding of the mechanisms of action of first-line drugs, IFNB,16 and glatiramer acetate.17
Understanding the Prognosis
An important objective in management is predicting the disease course in individual patients. For patients with CIS, the objective is to predict conversion to clinically definite MS. In patients with CIS suggestive of an MS attack and lesions on MR imaging, the likelihood of developing clinically definite MS is 88% during 14 years18 and 82% during 20 years.19 In established disease, objectives in management are to predict relapse in the short-term and predict disability and sustained disease progression in the long-term. A relapsing course is followed by sustained progression within 2 decades in 80% of cases.20
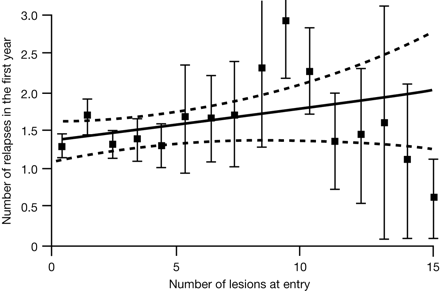
Disease progression is highly variable between individuals, reflecting the complex nature of the disease and variations in capacity for repair and compensation.21 Conventional MR imaging measures, including T2 lesion load, correlate poorly with clinical outcomes in MS,19,22 and correlations tend to weaken further in later stages of disease.23 Meta-analysis of the predictive value of gadolinium-enhanced MR imaging similarly indicates a low ability to predict relapses and development of impairment and disability (Fig 1).24
Predictive value of gadolinium-enhanced MR imaging for relapses in MS: a meta-analysis. Reprinted with permission from Lancet.24
A number of explanations have been suggested for why MR imaging assessments are dissociated from clinical status and the development of disability—the so-called “clinicoradiologic paradox”:
-
Deficiencies in clinical and MR imaging assessments.
-
The presence of strategic-versus-nonstrategic lesions.
-
The dual role of the immune system, both in destroying and promoting repair.
-
The role of neurodegenerative processes that gain importance as the disease progresses.
-
Abnormalities of apparently normal white and gray matter.
-
The role of adaptation and reorganization in compensating disease-related damage.
Consensus Statement.
More reliable and standardized approaches to MR imaging assessments in MS may lead to better correlation with clinical course. Until the reasons that underlie the clinicoradiologic paradox are fully identified, prognosis in individual patients cannot be based on MR imaging findings alone.
Monitoring Therapy
MR imaging is widely used to investigate the anti-inflammatory effects of therapies in clinical trials. In this setting, the most widely adopted and best-supported MR imaging assessments are T2 (for lesion load and new and enlarging lesions) and gadolinium-enhanced T1 (for total lesion number, new and enlarging lesions, and lesion load).
The Prevention of Relapses and Disability by Interferon-Beta1a Subcutaneously in Multiple Sclerosis trial included MR imaging assessments in patients with clinically definite or laboratory-supported relapsing-remitting MS. T2 and gadolinium-enhanced T1 MR imaging identified the onset of maximal therapeutic effect for IFNB-1a at 3 months.4 Other investigations, such as the fingolimod phase II study, similarly demonstrated the utility of MR imaging to monitor the effects of therapy in a trial setting.25 In confirmation of individual studies, a recent meta-analysis reported strong correlations between the effects of therapies on relapses and their influence on MR imaging activity.26
Less convincing evidence is available to support a role for other MR imaging measures in monitoring the effects of therapy, including T1 “black holes” (hypointense lesions) or atrophy of the brain or cord, and even less evidence supports a role for magnetization transfer MR imaging, DTI, spectroscopy, or functional MR imaging—though these remain areas of active investigation.27
Some centers routinely use MR imaging to monitor response to therapy in individual patients. If neurologists choose to use MR imaging to monitor a patient's response to therapy, a rational approach is baseline assessment with follow-up at 3 or 6 months and again at 12 months. Reassessment with MR imaging may be sooner if there are concerns about the patient's disease course. Stable MR imaging assessments in an individual with clinically silent disease supports continuation of the current treatment. Identification of new lesions in a clinically silent individual may indicate a change in therapy or the need for more frequent follow-up. A major increase in lesion number in a modestly clinically active patient or a patient with indeterminate findings indicates that therapy should be re-evaluated.
Consensus Statement.
MR imaging has utility for monitoring the effects of therapies in clinical trials. Further evidence is needed to support a role for MR imaging in monitoring therapeutic response in routine clinical practice.
Benefits of Early Therapy in MS
There is an encouraging trend toward initiating therapy early in the disease course, so now almost all patients with MS are treated following the first event. The rationale for early initiation of therapy is to reduce the frequency of relapses and slow progression to disability.
Outcomes from well-designed placebo-controlled trials of IFNB indicate that early treatment—at the first clinical demyelinating event—can slow progression to clinically definite MS.5,28–31 Serial MR imaging assessments, including T2 and gadolinium-enhanced T1 scans, supported clinical observations of improvement in these trials.
Most trials investigating the benefits of early treatment have been short-term. An exception is a large observational study of early treatment with IFNB in 1504 patients with relapsing-remitting MS who were followed for 7 years.32 Patients treated with IFNB showed significant reductions in secondary progression compared with a placebo, and the authors concluded that early treatment slowed long-term progression of MS.
Consensus Statement.
Early initiation of treatment offers benefits in most patients, and these benefits appear to persist for the long-term. MR imaging contributes to the early initiation of treatment by facilitating early diagnosis.
Techniques and Protocols for MR Imaging in MS
Conventional MR imaging
Conventional MR imaging is a reliable and accurate diagnostic technique, providing positive findings in approximately 95% of patients with clinically definite MS. MR imaging is widely recognized as superior to other imaging modalities, including CT, for the visualization of lesions, particularly smaller lesions, and has largely replaced alternative imaging techniques in practice.
MS plaques can be characterized at MR imaging by their location, morphology, signal intensity, and degree of gadolinium enhancement. Acute-phase plaques appear as rounded areas of high-signal intensity on T2 sequences. Gadolinium enhancement on T1 sequences is related to BBB damage associated with inflammation. There are 2 patterns of enhancement: uniform enhancement, reflecting the onset of a new lesion, and ringlike enhancement, indicating reactivation of an older lesion.33 Nonenhancing lesions are the result of earlier episodes of disease. T2-weighted MR imaging is considered the most sensitive diagnostic test for demonstrating disease dissemination, but with moderate specificity. T1-weighted gadolinium-enhanced imaging offers increased specificity by differentiating enhancing from nonenhancing lesions. Use of both of these imaging techniques provides optimal specificity.34
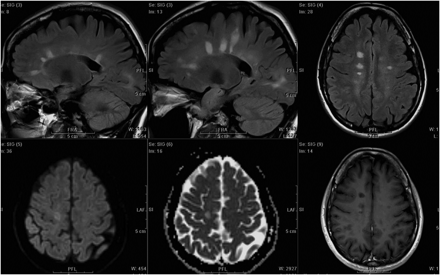
Typical MR imaging findings that are sensitive and specific for diagnosing MS include plaques along callososeptal interfaces and perivenular extension (Dawson finger) (Fig 2).35 Atypical MR imaging findings in MS include lesions that mimic tumors and autoimmune vasculitis. In these cases, characteristic differences in lesion distribution, supported by clinical and laboratory investigations, assist differential diagnosis. The revised McDonald criteria include recommendations for excluding alternative diagnoses through history, clinical evaluation, and appropriate laboratory studies,2 while “red flags” have been developed to alert clinicians to reconsider the differential diagnosis more extensively in clinically suspected MS.36
Typical MS with brain lesions.
Besides visualizing lesions in the brain, MR imaging may be used to image lesions associated with optic neuritis, neuromyelitis optica, and spinal cord MS. Optic neuritis is present in ≤50% of patients with MS and is frequently the presenting sign. Gadolinium enhancement is a sensitive method for visualizing optic neuritis and has a role, along with brain MR imaging and symptoms, in establishing a definitive diagnosis.37
The spinal cord is also frequently involved in MS and, for most patients, both spinal cord and the brain are affected.38 In ∼25% of patients, however, lesions are present in the spinal cord alone.33 Most spinal lesions are localized to the cervical rather than the thoracic cord and tend to be multifocal and asymmetric.39 At MR imaging, spinal lesions show increased T2 signal intensity and, frequently, gadolinium enhancement. In general, findings at spinal MR imaging are less definitive compared with brain MR imaging for diagnosing MS.
Indications and protocols for suspected spinal MR imaging remain an area of debate. Recommendations at the expert meeting for use of MR imaging in patients with and without a spinal presentation are summarized in Recommendation 4.
Consensus Statement.
MR imaging is the optimal radiologic technique for supporting a diagnosis of MS in the brain and spinal cord. Full exchange of imaging and clinical information between radiologists and neurologists is essential for reaching a correct diagnosis.
Role of Contrast Enhancement
The meeting participants noted a lack of expert guidance on the role of contrast agents in MS and included extensive discussion on this topic. Estimates suggest that ≥35% of MR imaging examinations are performed with contrast agents, usually gadolinium-containing agents. Contrast enhancement in MS increases the reliability of MR imaging to depict active lesions34 and has a pivotal role in demonstrating dissemination in time, as defined in the revised McDonald criteria.2 Contrast enhancement also assists in excluding confounding diagnoses, including other inflammatory conditions and tumors. For these reasons, gadolinium enhancement is widely recommended for the diagnosis and initial evaluation of MS.6 At some centers, contrast-enhanced MR imaging is additionally performed to monitor the effects of therapy.
Consensus Statement.
Contrast enhancement by using gadolinium-containing agents increases the efficacy of MR imaging of MS lesions.
Characteristics of Contrast Agents
A number of gadolinium-containing contrast agents are available for use in MR imaging, including gadobenate dimeglumine (MultiHance; Bracco, Milan, Italy), gadobutrol (Gadovist; Bayer Schering Pharma, Berlin-Wedding, Germany), gadodiamide (Omniscan; Nycomed Amersham, Oslo, Norway), gadofosveset trisodium (Vasovist; EPIX Pharmaceuticals, Lexington, Massachusetts), gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany), gadoterate meglumine (Dotarem; Guerbet, Aulnay-sous-Bois, France), gadoteridol (ProHance, Bracco), gadoversetamide (OptiMARK; Mallinckrodt, St Louis, Missouri), and gadoxetic acid disodium (Primovist, Bayer Schering Pharma). Most currently available gadolinium-containing contrast agents are at a concentration of 0.5 mol/L, while gadobutrol is formulated at a higher concentration of 1.0 mol/L.40
Stability.
Gadolinium-containing contrast agents can be classified by the molecular structure of their gadolinium-chelate complex—whether macrocyclic or linear—and, within the linear class, by whether they are ionic or nonionic.
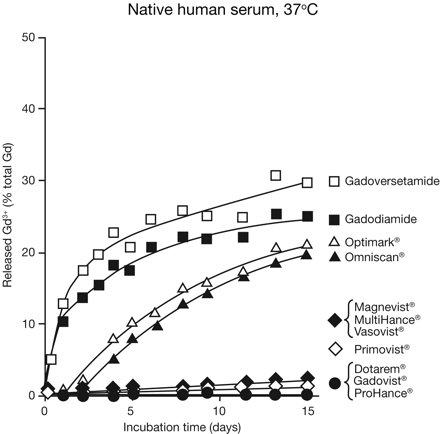
Contrast agents with macrocyclic structures demonstrate increased stability and a lower propensity to release gadolinium ions compared with linear contrast agents.41 This was confirmed in a recent study comparing the stability of contrast agents in human serum under physiologic conditions.42 Release of gadolinium ions was substantially reduced for macrocyclic agents—gadobutrol, gadoteridol, and gadoterate meglumine—relative to agents tested with linear structures (Fig 3). The study also found that the addition of phosphate to the serum at a concentration of 10 mmol/L (to simulate end-stage renal disease) accelerated the release of gadolinium ions from nonionic linear agents and, to a lesser degree, from ionic linear agents, but that macrocyclic agents remained stable.
Comparison of rates of Gd3+ release for 1 mmol/L solutions of gadolinium-containing contrast agents in native human serum from healthy volunteers at 37°C. Reprinted with permission from Frenzel T, Lengsfeld P, Shirmer H, et al. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37°C. Invest Radiol 2008;43:817–28.
Release of gadolinium ions from contrast agents may be relevant to the development of NSF. NSF is a rare outcome in patients with severe kidney failure, characterized by thickening, induration, and hardening of the skin. Some workers attribute NSF to gadolinium ions released from contrast agents.43 In support of this still-debated association, clinical reports suggest that NSF is associated most commonly with nonionic linear contrast agents and rarely with agents with macrocyclic structures.44
Contrast Dose and Characteristics.
Although there is consensus on the benefits of gadolinium-based agents in MR imaging, debate continues over how best to use these agents to optimize lesion visualization in MS.45
The standard dose of contrast agent for MR imaging of the CNS is 0.1 mmol per kilogram of body weight, and this dose is sufficient for diagnosis in most patients. Studies investigating a range of pathologies, including brain tumors, gliomas, and MS, indicate, however, that lesion detection may be improved further with higher concentrations (0.2–0.3 mmol/kg).46–50 These higher concentrations may have a role in cases of diagnostic doubt following the standard 0.1 mmol/kg dose. As an alternative to administering a contrast agent in sequential doses, a dose-comparison study of gadobutrol in MS recommended using a double dose (0.2 mmol/kg) at the initial assessment, an approach that was endorsed by expert panel experience. A single injection may offer optimal balance in terms of sensitivity, time, costs, and detection of active lesions.51
A physicochemical property of contrast agents relevant to imaging performance is relaxivity, which defines the ability of an agent to alter tissue relaxation rates. Complementing theoretic studies, higher relaxivity relates to increased imaging performance in clinical trials comparing gadolinium-containing contrast agents.52–55 Gadobutrol has a higher relaxivity than other macrocyclic agents currently available, leading to the highest T1 shortening per volume.52,56
Acquisition Protocol by Using Contrast Agents.
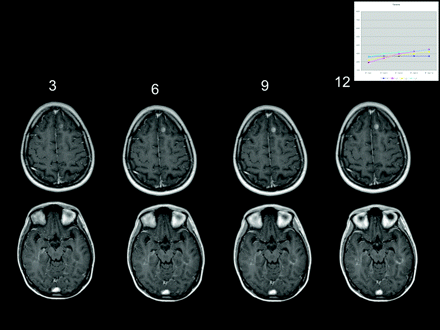
Another consideration for optimizing lesion enhancement is the timing of image acquisition following contrast agent injection.45 A recent study reported that the sensitivity of MR imaging to detect active MS lesions was progressively enhanced at up to between 5 and 10 minutes postinjection of gadobutrol,47 which was supported by case study experience among expert meeting participants (Fig 4). Although increasing enhancement over time is an area for further investigation, the meeting participants agreed that not all contrast agents share the characteristic of gadobutrol of progressive enhancement postinjection.
Case study shows brain lesion enhancement with gadobutrol. Images were obtained at 3, 6, 9, and 12 minutes postinjection.
Consensus Statement.
Complex stability is an important safety consideration when selecting gadolinium-containing contrast agents, especially in patients with renal disease. Physicochemical characteristics (including concentration and relaxivity) and acquisition protocol influence imaging performance. Gadobutrol meets the criterion of high complex stability and provides the highest T1 shortening with high image quality.
Acquisition Protocols for MR Imaging
Acquisition protocols for MR imaging in MS vary widely between centers, reflecting practitioner preference and local availability of equipment. A comprehensive acquisition protocol may include a localizer scan, FLAIR sagittal, T2 and FLAIR axial, pre- and postcontrast T1 axial, and (optionally) DWI and 3D-T1-weighted spoiled gradient recalled-echo.
Simpler protocols offer time and cost savings relative to more comprehensive protocols, can be standardized across centers, and are likely to diagnose 90% of MS cases. A simple protocol was recommended by expert meeting participants:
-
Dual-echo and FLAIR, axial whole brain (to detect gray matter lesions)
-
Optional dual-echo or FLAIR, sagittal midline (to detect corpus callosum lesions)
-
Skip unenhanced T1 (provides little additional information)
-
Contrast-enhanced T1 scan
-
Optional DWI (to differentiate other diagnoses).
A standard dose of contrast agent (0.1 mmol/kg) should be injected before the first MR image. A scanner with at least 1T optimizes image quality and tissue contrast.
Consensus Statement.
A simple acquisition protocol that can be standardized across centers offers advantages for diagnosing MS. A gadolinium-based contrast agent is recommended for all diagnostic procedures.
Novel MR Imaging Techniques
Conventional MR imaging is associated with shortcomings including low sensitivity to gray matter lesions and diffuse white matter involvement and a low capacity to predict clinical status.3 Newer uses of existing MR imaging techniques, the availability of novel contrast agents (eg, high molar agents and smart nanoparticles), and emerging techniques (eg, MR spectroscopy, DWI, DTI, perfusion-weighted imaging, and permeability testing of the BBB) offer opportunities for improved specificity and sensitivity in diagnosing and monitoring MS.3,33
Consensus Statement.
Novel MR imaging techniques are continuously being developed and appraised for roles in the management of MS. Outside expert centers, T2 and gadolinium-enhanced T1 MR imaging remain mainstay approaches in practice.
Summary of Expert Meeting Recommendations
From discussion during the expert meeting, the participants summarized 5 key recommendations for MR imaging in MS.
Recommendation 1: Diagnosis Versus Monitoring
-
Applications of MR imaging in MS should distinguish diagnosis from monitoring.
-
MR imaging, especially used with contrast agents, has an important role in diagnosing MS, excluding alternative diagnoses, and characterizing dissemination in space and time according to the revised McDonald criteria.
Recommendation 2: The Clinical–MR Imaging Paradox
-
MR imaging is currently not reliable for predicting the clinical evolution of MS.
-
Clinical decisions should not be based solely on the presence of lesions detected at MR imaging.
Recommendation 3: Importance of the McDonald Criteria
-
Standardized protocols and reporting procedures should be uniformly adopted on the basis of the revised McDonald criteria.
-
This message should be communicated widely to radiologists and neurologists at congresses and other educational opportunities.
-
The McDonald criteria need to be adapted for Asian populations.
Recommendation 4: Brain-Versus-Spinal Cord MR Imaging
-
For nonspinal cord presentation, brain MR imaging should be performed. MR imaging investigation may be stopped if there are sufficient lesions to support dissemination in space. If that is not the case, additional spinal MR imaging may be diagnostically helpful.
-
For spinal cord presentation, start investigations with spinal cord MR imaging, mainly to exclude alternative conditions. If MS remains suspected, perform brain MR imaging to identify additional lesions.
Recommendation 5: A Simple Standardized Protocol
-
A simple standardized MR imaging protocol should be implemented across centers.
-
Dual-echo and FLAIR axial whole brain, precontrast dual-echo or FLAIR sagittal midline (optional), and contrast-enhanced T1 scanning should be performed.
-
A scanner with at least 1T should be used.
-
Use specific landmarks to achieve consistent section positioning, especially for serial studies.
-
-
A gadolinium-containing contrast agent should be used for all diagnostic procedures.
-
Inject a standard dose of contrast agent (0.1 mmol/kg) before the first MR imaging.
-
For some contrast agents, there is evidence of a progressive increase in lesion-detection rate due to delayed enhancement.
-
Consider high signal intensity and safety as well as complex stability when selecting the contrast agent.
-
Acknowledgment
PAREXEL MMS provided editorial support.
Footnotes
-
The expert meeting and the preparation of this review article were funded by an unrestricted educational grant from Bayer Schering Pharma AG.
Indicates open access to non-subscribers at www.ajnr.org
References
- Copyright © American Society of Neuroradiology