Abstract
BACKGROUND AND PURPOSE: CM-AVM is a recently recognized autosomal dominant disorder associated with mutations in RASA1. Arteriovenous lesions have been reported in the brain, limbs, and the face in 18.5% of patients. We report a novel association between RASA1 mutations and spinal arteriovenous anomalies.
MATERIALS AND METHODS: In a collaborative study, 5 index patients (2 females, 3 males) with spinal AVMs or AVFs and cutaneous multifocal capillary lesions were investigated for the RASA1 gene mutation.
RESULTS: All 5 patients were found to have RASA1 mutation (2 de novo, 3 familial), and all had multifocal capillary malformations at birth. Neurologic deficits developed at ages ranging from infancy to early adulthood. All spinal anomalies (2 AVMs at the conus, 1 AVM at the lumbosacral junction, and 1 cervical and 1 cervicothoracic AVF) were complex, extensive, and fast-flow lesions. All patients required treatment based on the clinical and/or radiologic appearance of the lesions.
CONCLUSIONS: To our knowledge, an association of RASA1 mutation and spinal AVM/AVF has not been described. MR imaging screening of patients with characteristic CMs and neurologic symptoms presenting at a young age may be useful in detecting the presence of fast-flow intracranial or intraspinal arteriovenous anomalies before potentially significant neurologic insult has occurred.
Abbreviations
- ASA
- anterior spinal artery
- AVF
- arteriovenous fistula
- AVM
- arteriovenous malformation
- CM
- capillary malformation
- GTP
- guanosine 5′-triphosphate
- HHT
- hereditary hemorrhagic telangiectasia
- n-BCA
- n-butyl 2-cyanoacrylate
- R
- right
- SWS
- Sturge-Weber syndrome
CM-AVM is a newly recognized autosomal dominant disorder caused by mutations in the RASA1 gene.1,2 The RASA1 gene encodes the protein activator 1 (p120-RasGTPase-activating protein), which is essential for the organization of endothelial cells into highly organized networks,1 switching the active GTP-bound Ras to the inactive GDP-bound form. Murine RASA1 knockout embryos exhibit abnormal vascular development.3,4 More than 18% (18.5%) of patients with a RASA1 mutation have a fast-flow anomaly such as an AVM or AVF in the brain (7.1%), face (7.8%), or limbs (3.6%).2,5 Previously reported intracranial arteriovenous lesions in CM-AVM are typically macrofistulous, usually presenting with neurologic signs at birth or before 1 year of age.3 Small, usually multifocal, and randomly distributed cutaneous capillary malformations are the most prominent feature in patients with RASA1 mutations.3 Atypical features of these cutaneous lesions can lead to further diagnostic evaluation for fast-flow anomalies even before these become clinically manifest. As an interdisciplinary and multi-institutional collaboration, the authors identified 5 patients with RASA1 mutations and fast-flow anomalies in the spine, to our knowledge, a previously undocumented association.
Materials and Methods
Data from patients were collected from 8 centers located in Europe and North and South America. All 5 patients in the series or their legal guardian provided written informed consent according to a protocol approved by the institutional review board committee of Children's Hospital Boston, Harvard Medical School, Boston, and the ethics committee of the medical faculty of Université catholique de Louvain, Brussels, Belgium. Blood was drawn from each participant for molecular genetic screening performed at the Laboratory of Human Molecular Genetics, de Duve Institute, Université catholique de Louvain. A clinical questionnaire was completed by each referring physician that included extent, location, and temporal course of the cutaneous changes, the onset and severity of neurologic symptoms, the radiologic features of the vascular anomalies, and treatment.
Results
Clinical Features
All patients had capillary malformations at birth (Fig 1); these were multifocal in 3 of the 5 patients. Neurologic symptoms occurred at 16 months of age in 1 patient,6 2 years of age in 1 patient, 4 years of age in 1, 6 years of age in 1, and at 23 years of age in 1 patient. In the 2 cases of upper spine AVF, the patients presented with relatively nonspecific symptoms, such as headache. Patients with lower spine lesions presented with severe sensorimotor deficits and flaccid paralysis with neurogenic bladder, as described in the Table.
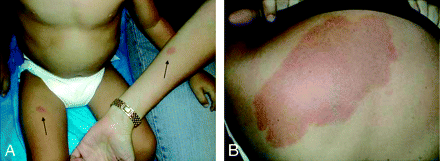
Atypical capillary malformation on the patient's thigh and the mother's forearm (A) and the mother's back (B). The patient's uncle had a history of pontine hemorrhage from brain AVM, and genetic screening for a RASA1 mutation was positive in this patient
Collaborative collection of patients
Genetic Testing
Two patients were found to have a de novo mutation in the RASA1 gene; the other 3 patients had familial RASA1 mutations (Table). Only 1 patient (case 3 in the Table) was diagnosed with RASA1 mutation on the basis of his atypical capillary malformations before he became neurologically symptomatic with severe headaches and intermittent motor tics involving repetitive jaw thrusting.
Radiographic Appearance, Treatment, and Outcome
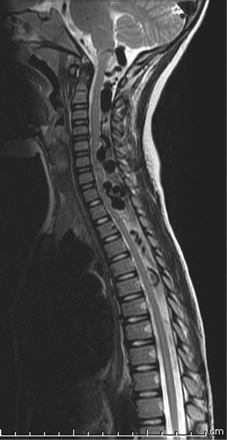
One patient presented with headaches and motor tics (case 3 in the Table). Given the known presence of the RASA1 mutation in this patient (Fig 1), there was a low threshold for obtaining cross-sectional imaging, and brain and spine MR imaging revealed marked ectasia of the deep venous system of the posterior fossa and posterior intradural space (Fig 2). Subsequent cerebral and spinal angiography revealed a fast-flow posterior spinal artery pial AVF, and combined endovascular/surgical treatment was undertaken (Figs 3⇓⇓–6).
Sagittal T2-weighted image of the cervical and upper thoracic spine. Ectatic veins in the posterior intradural space extend from the cervicomedullary junction to T6, causing cervical and thoracic cord compression.
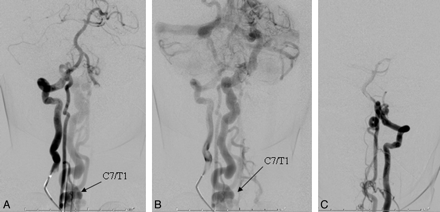
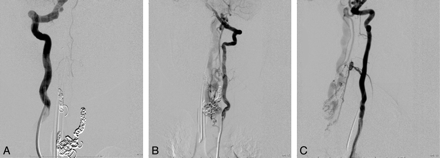
A, In a frontal view of the early arterial phase, a right vertebral artery injection is seen to opacify a markedly enlarged right posterior spinal artery arising from the right lateral spinal artery and ending in a direct AVF into an ectatic venous pouch at C7-T1. B, Slightly delayed frontal view of the right vertebral artery injection shows rostral reflux of arterialized blood up to the posterior fossa, with marked enlargement of the lateral brain stem venous system. C, Frontal view of a left vertebral artery injection demonstrates a near mirror-image configuration, with opacification of the AVF via the left lateral spinal/posterior spinal artery.
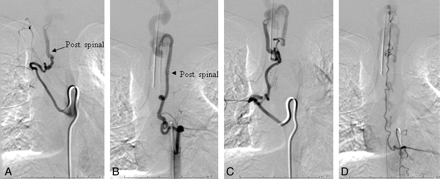
A, Injection of the right T7 segmental artery reveals brisk opacification of the AVF via a right posterior spinal branch supplying the fistula from below at the right inferolateral aspect of the venous pouch. B and C, Supply from below also comes from the left T7 (B) and right T8 (C) segmental arteries. D, Injection of the left T9 segmental artery opacifies the ASA axis, with the artery of Adamkiewicz originating at this level. The ascending ASA axis is enlarged and provides collateral supply to the posterior spinal AVF, via sulcocommissural branches.
A, Following coil embolization, there is no further opacification of the fistula from the right vertebral arterial injection. A−C, Segmental branches of the left vertebral artery continue to opacify the AVF, albeit less briskly, as seen in frontal (A) and lateral views (C).
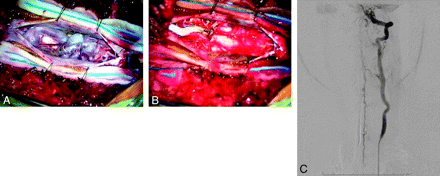
Intraoperative photographs via the operating microscope after opening the dura. A and B, Prominent dilated veins on the dorsal surface of the cord with evidence of coils in a large venous varix are seen preresection (A) and are absent postresection (B). C, Frontal view of a left vertebral artery injection on postoperative angiography demonstrates complete elimination of the AVF. The patient is neurologically intact.
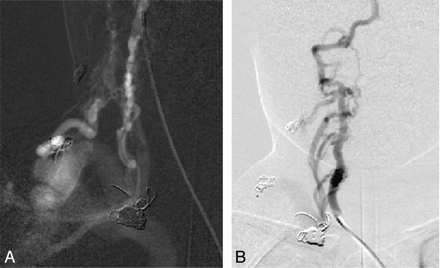
In the other patient (case 2 in the Table) with cervical AVF, the right vertebral artery and thyrocervical trunk provided the main supply to a complex AVM, which was treated by endovascular methods only (Fig 7). The patient had a good recovery.
Frontal views of a right subclavian (A) and right vertebral artery (B) injection during embolization demonstrate a complex AVF supplied by branches of the right thyrocervical trunk and segmental right vertebral artery branches.
In the 2 patients with flaccid paralysis and bladder dysfunction, a fast-flow AVM was identified at the conus, treated surgically in 1 patient and via n-BCA embolization in the other. The fifth patient, with severe sensorimotor deficits, had a fast-flow AVM at the lumbosacral junction, which was treated with glue embolization. All arteriovenous lesions demonstrated a fast-flow pattern on angiography.
Discussion
CM presents as a characteristic macular stain and has a reported incidence of 0.3% in neonates.7 This slow-flow lesion consists of dermal capillary venular-like channels that are dilated and/or increased in number.7 There are reports of a familial tendency for CM. In a study from the Division of Plastic and Reconstructive Surgery of the Academic Medical Center, Amsterdam, 55 of 280 new patients (19.6%) presenting for treatment had an affected relative.8 When these pedigrees were analyzed, there was no discernable mode of inheritance. While the most common presentation of CM is without association with other anomalies, in some patients, CM can herald the presence of an underlying anomaly of the neurovascular axis.
CM-AVM is a newly described syndrome caused by heterozygous mutations in RASA1, located on 5q13.3.1 The capillary stains in this disorder are phenotypically slightly different from the common-variety CM. They are round or oval and pinkish-tan.9 When solitary, they range in diameter from very small to several centimeters. Multiple lesions also occur and may continue to appear throughout childhood. Often these lesions are surrounded by a pale halo, and even a small stain can exhibit a prominent to-fro murmur by hand-held Doppler examination.9 These atypical cutaneous CMs occur in association with fast-flow anomalies, namely AVM and AVF, in approximately 18.5% of affected families.1,9 They involve the cutis, subcutis, muscles, or bones. Intracranial manifestations include vein of Galen malformations with symptoms at birth or pial AVMs or AVFs, which become symptomatic during the first year of life.9
To the best of our knowledge, an association of CM-AVM and spinal arteriovenous anomalies has not been described. Macro-AVFs are fast-flow direct shunts, fed by 1 or multiple spinal cord arteries, typically ending directly in a giant venous ectasia (characteristic of these lesions), with secondary drainage into the extrinsic and intrinsic venous system of the cord.10 Progressive symptoms are a result either of congestion in the intrinsic and extrinsic venous system of the cord or of direct mass effect inflicted by the ecstatic veins. Bleeding from spinal AVFs is seen more frequently in children than in adults.10 The complexity of these lesions often mandates multidisciplinary treatment, as shown in Figs 3⇑⇑–6.
SWS is the most well-known association of facial CM, often with diffuse capillary staining of the trunk and extremities and ipsilateral vascular anomalies of the leptomeninges and ocular choroid.11,12 In SWS, a metameric distribution of the facial and cerebral venous lesions is seen, which is rarely bilateral. A faint upper facial CM may also indicate a unilateral AVM of the retina and intracranial optic pathway, an entity known by the syndromic terms Bonnet-Dechaume-Blanc in the European literature and Wyburn-Mason in the English literature.13 This, too, is a metameric syndrome affecting the cerebrofacial territory, giving rise to cerebral and facial AVMs. A circular capillary stain of the occipital scalp is often present with heterotopic neural nodules and the “hair collar” sign.14 Enjolras et al15 reported skin anomalies in the cervical area to be indicative of occult spinal dysraphism. CM on the posterior thorax may be a sign of an underlying AVM of the spinal cord.16,17 Cobb syndrome is the spinal expression of the metameric AVM syndrome involving skin, bone, and spinal cord. A capillary stain over the lower lumbar region may overlie spinal dysraphism, lipomeningocele, tethered spinal cord, and diastematomyelia.18
Intracranial AVMs in HHT are usually diagnosed in adulthood, though some pediatric cases have been reported.19 In contrast to CM-AVM, the liver and lungs are typically involved. The mucocutaneous stains of HHT are radiating or arborizing telangiectasias. Macrofistulous spinal cord AVMs have also been reported in patients with HHT.10,20,21 Definite diagnosis of HHT is made clinically if 3 of the 5 Curaçao criteria, established in June 1999 by the Scientific Advisory Board of the HHT Foundation International, are met. The criteria include epistaxis, telangiectasias, visceral AVMs, cerebral or spinal AVMs, and a first-degree relative with HHT.22
Fast-flow fistulas, both intracranial and in the spinal axis, should be considered in patients with the characteristic CMs, who evidence even minor neurologic symptoms. Once fixed neurologic deficits are manifest, functional recovery is much less likely.23 The availability of genetic testing will likely result in an increased reporting incidence of CM-AVM, with 200 individuals thus far identified with RASA1 mutations.9 It is likely that some patients with arteriovenous anomalies of the neural axis, previously labeled as Cobb syndrome or HHT, might, in fact, be more accurately diagnosed with CM-AVM.
Footnotes
-
Indicates Editor's Choices selection
-

References
- Received July 20, 2009.
- Accepted after revision September 9, 2009.
- Copyright © American Society of Neuroradiology