Abstract
SUMMARY: SIFs are a common, though often unsuspected, cause of low back pain in the elderly. Although numerous radiographic modalities can be used to diagnose SIFs, bone scintigraphy and MR imaging are the most sensitive. Conservative management involves various combinations of bed rest, rehabilitation, and analgesics. More recently, sacroplasty has emerged as an alternative therapy for the treatment of SIFs, with prospective studies and case reports suggesting that it is a safe and effective therapy. This article reviews the imaging appearance of SIFs and discusses treatment options with a focus on sacroplasty.
Abbreviations
- FEA
- finite-element analysis
- MDP
- methylene diphosphonate
- PMMA
- polymethylmethacrylate
- SIF
- sacral insufficiency fracture
- VAS
- visual analog pain scale.
SIFs are a common cause of debilitating back pain in the elderly. Since they were first described as a clinical entity in 1982 by Lourie,1 the awareness of this entity among health care professionals has increased. However, there is often a delay in diagnosis because clinical symptoms are frequently vague and nonspecific and can mimic a variety of pathologic processes in a predominantly elderly population, including radiculopathy and metastatic disease. Bone scintigraphy and MR imaging are the most sensitive studies to detect SIFs, though findings have been described in a wide variety of radiologic modalities. The standard of care for the treatment of SIFs has been conservative management, with variable courses of bed rest, rehabilitation, and analgesics prescribed.2–4 More recently, sacroplasty, a minimally invasive procedure akin to vertebroplasty in the thoracolumbar spine, has been advocated as an alternative to conservative therapy. Retrospective case series and prospective studies suggest that sacroplasty is a safe and effective procedure, providing early symptomatic relief in patients with SIFs.5–8 This article discusses the imaging findings and treatment of SIFs.
SIFs commonly affect elderly women with osteoporosis,9,10 though other reported risk factors include pelvic radiation, steroid-induced osteopenia, rheumatoid arthritis, multiple myeloma, Paget disease, renal osteodystrophy, and hyperparathyroidism.2,9,11 Of these, osteoporosis is the most prevalent, and almost all patients with SIFs will demonstrate severe osteopenia on dual x-ray absorptiometry, even if other risk factors are present.2 Prior pelvic radiation is another well-established risk factor for the development of SIFs, with a reported prevalence of 21%–34%.12,13 The incidence may even be as high as 89%, as suggested by a prospective study of patients undergoing pelvic radiation for cervical cancer.14
Almost all patients with SIFs are older than 55 years of age, with a mean age between 70 and 75 years in most studies.4,6,15,16 The true incidence of SIFs is unknown but has been reported to be between 1% and 5% in at-risk patient populations.17–19 Antecedent trauma is not identified in two-thirds of patients3,4 and, when present, is usually minor.16
Patients with SIFs most commonly present with diffuse low back pain, which may radiate to the buttock, hip, or groin.3,15,20 Patients may have some tenderness to palpation in the lower back and sacral region, though this is not a consistent finding.15 Neurologic symptoms related to SIFs are unusual, though may be seen in 5%–6% of patients, most commonly manifesting as a sacral radiculopathy.2 However, a case of cauda equina syndrome related to SIFs has been reported.21
SIFs can occasionally be confused with metastatic disease, both clinically and on imaging studies,22 resulting in unnecessary work-up and biopsy.9–10 This is frequently a confounding factor in elderly patient populations, many of whom have a known primary malignancy or are being evaluated for an occult tumor.16,22,23 In fact, approximately 45% of patients with SIFs have a history of malignancy.2
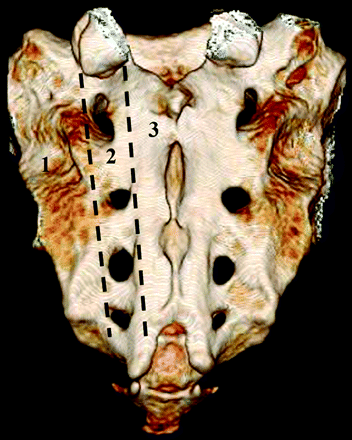
Insufficiency fractures are a subtype of stress fracture that results from normal stress applied to abnormal bone that has lost its elastic resistance. Bone insufficiency is often the result of osteoporosis or other metabolic bone disease, though osseous metastatic disease and marrow replacement processes can also cause insufficiency fractures. SIFs most commonly involve the sacral ala, lateral to the neural foramina and medial to the sacroiliac joints (zone 1) (Fig 1).24 Fractures may be unilateral or bilateral and are reported with relatively equal frequency in the literature.2,9 There may also be a horizontal component to the fracture through the sacral bodies. This unique fracture pattern may be related to axial loading and weight-bearing transmitted through the spine, resulting in sacral alar strain.25,26 Additionally, osteoporosis causes asymmetric loss of bony trabeculae in the sacral ala compared with the vertebral bodies, placing the lateral aspect of the sacrum at increased risk of insufficiency.27
Posterior projection from a 3D volume rendered normal sacrum. Labels denote the classification system proposed by Denis et al.24 Zone 1 contains the sacral ala and portions of the sacrum lateral to the neural foramina. Zone 2 contains the foramina. Zone 3 contains the sacral bodies.
Of note, there is a high incidence of concomitant pelvic insufficiency fractures, and radiologists should be aware of this association. SIFs are most frequently associated with insufficiency fractures of the pubic rami and parasymphyseal region, with a reported coincidence of 88%.9 Associated insufficiency fractures have also been described in the superior acetabulum and iliac wing.28,29 It has been suggested that the sacrum is the initial site of failure and that this results in increased stress on the rest of the bony pelvis.9
Anatomy
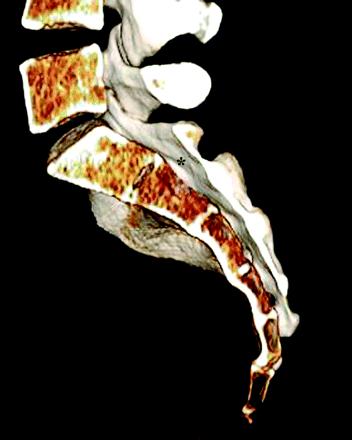
The sacrum is a triangular or shield-shaped bone at the caudal end of the spine comprising 5 vertebral segments. Denis et al24 divided the sacrum into 3 zones (Fig 1). Zone 1 is composed of the sacral ala and bone between the sacroiliac joints and the neural foramina and is the most common site of SIFs. Zone 2 contains the neural foramina, and Zone 3, the sacral bodies. The sacrum articulates with the iliac bones of the pelvis laterally, the lumbar spine superiorly, and the coccyx caudally. The sacral spinal canal extends along the length of the dorsal aspect of the sacrum (Fig 2). Of note, the thecal sac usually ends at S2.
Lateral slab projection of a 3D volume rendered normal sacrum demonstrating the relationship of the central sacral canal (asterisk) in the sacrum.
Imaging
The imaging findings of SIFs have been well described in the literature and are reviewed below. However, initial imaging is often not targeted at the sacrum but rather at the lumbar spine and/or pelvis. In fact, there is often a delay of 40–55 days from symptom onset before dedicated sacral imaging is pursued.28
Plain Films
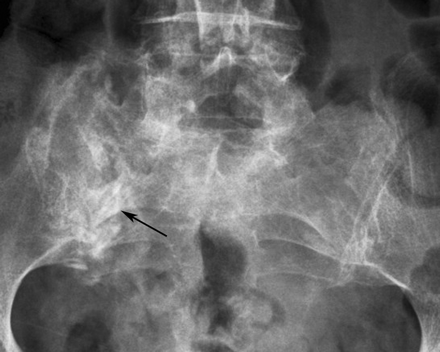
SIFs usually appear as vertical bands of sclerosis oriented parallel to the sacroiliac joints in zone 1 of the sacrum (Fig 3).29,30 Occasionally, cortical disruption and/or fracture lines may be evident.31 In one meta-analysis, sclerosis was reported in 57% of patients, with a fracture line evident in only 12.5%.2 On follow-up imaging, the time course of radiographic resolution of sclerotic fracture lines is quite variable, ranging between 1 and 13 months.9
Coned-down anteroposterior radiograph of the lumbar spine demonstrates a right-sided SIF (black arrow) manifest as a band of sclerosis and a cortical break in the lateral aspect of the sacrum.
Occasionally fractures can have an aggressive appearance, simulating malignancy, with areas of sclerosis and periosteal reaction. This may lead to unnecessary work-up and biopsy, which can also be confused with an aggressive process pathologically.9,31
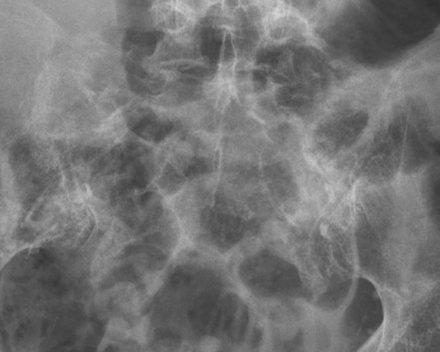
Plain films are, therefore, insensitive for the detection of SIFs, and dedicated radiographs of the sacrum are rarely acquired. More often, radiographs of the lumbar spine and pelvis are acquired in the initial work-up. Studies suggest that only 20%–38% of SIFs and pelvic ring fractures are identified on plain films,2,4 and these are often overlooked prospectively. Even when evaluated retrospectively, less than 50% of SIFs diagnosed on bone scintigraphy were evident on plain films.32,33 SIFs may not be evident in a large percentage of patients because of a high prevalence of osteopenia and obscuration by overlying bowel gas (Fig 4).31
Bowel gas obscures a left SIF in this patient with osteoporosis on this coned-down anteroposterior radiograph of the pelvis.
Nuclear Medicine
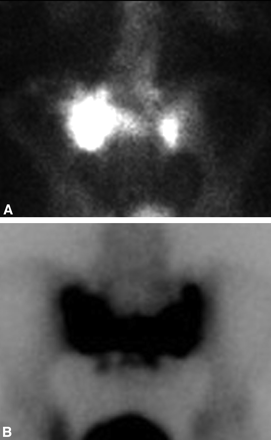
Bone scintigraphy with technetium Tc99m−labeled MDP is one of the most sensitive examinations for the detection of SIFs, and the imaging findings have been well documented.2,33 Various patterns of radiotracer uptake have been described, with the so-called “Honda” sign or H-pattern considered diagnostic of SIFs in the correct clinical setting (Fig 5).33 However, this pattern of radiopharmaceutical uptake is seen only in 20%–40% of patients.4,31 Variations in the pattern of radiopharmaceutical activity in SIFs include uptake oriented unilaterally in the sacral ala, unilaterally with a horizontal strut, bilaterally without a horizontal strut, and as multiple foci of activity.34 Posterior planar images are the most sensitive (ie, when the sacrum is closest to the detector).29 Follow-up scintigraphic results are quite variable, with changes in the radiopharmaceutical uptake pattern during a 10- to 33-month interval, ranging from resolution of abnormal activity to no change or even worsening activity.3,9,22,31
A, Posterior planar scintigraphic image from a Tc99m-MDP bone scan demonstrates asymmetric sacral uptake without a horizontal strut in the setting of SIF. B, Tc99m-MDP bone scan on a different patient demonstrates the classic Honda sign characteristic of SIFs. Note, incidentally, physiologic activity within the bladder in both cases.
Bone scintigraphy has a reported sensitivity of 96% for the detection of SIFs, with a positive predictive value of 92%.34 Some authors have suggested that characteristic patterns of uptake in the sacrum should be considered diagnostic in the correct clinical context, especially if there are no other sites of abnormal activity in the skeleton and no history of primary malignancy.33 However, there have been case reports of isolated metastases presenting as unilateral sacral uptake.34
CT
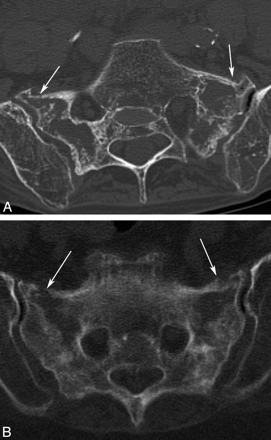
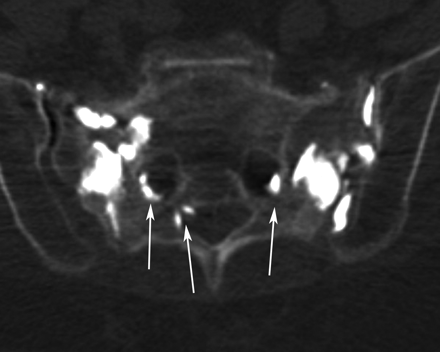
Similar to radiographs, CT may demonstrate sclerosis in the sacral ala and lateral sacrum paralleling the sacroiliac joints. Fracture lines with or without bony callous are evident in approximately 75% of patients (Fig 6).2 Fracture lines are usually sagittally oriented and are readily apparent on axial CT images; however, the full extent of the sacral fracture may not be evident if there is an isolated or significant horizontal component. For this reason, coronal images, either reformatted or directly acquired, may be helpful to visualize the full extent of the fractures.3
Axial CT scans of the pelvis in 2 different patients demonstrate bilateral SIFs (white arrows) with mottled sclerosis/lucency and cortical breaks.
CT is not as sensitive for the detection of SIFs when compared with bone scintigraphy or MR imaging, with a reported sensitivity between 60% and 75%.2,35 However, CT may be helpful to confirm inconclusive or equivocal findings on bone scintigraphy or MR imaging.9,31 Visualization of bone detail on CT may also be useful to determine if the fracture lines extend into the neural foramina, creating a potential pathway for cement if sacroplasty is being considered as a treatment option.36 CT can also be especially helpful when trying to differentiate fracture from metastatic disease because it can depict cortical destruction and/or a soft-tissue mass in cases of tumor involvement.3,12,37
MR Imaging
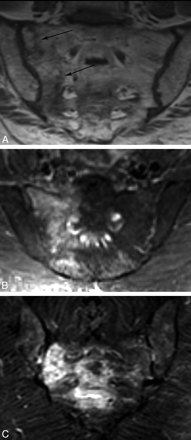
MR imaging can detect early changes of sacral insufficiency and, similar to bone scintigraphy, has a reported sensitivity at or near 100%.35 T2 short τ inversion recovery sequences are exquisitely sensitive for the detection of early marrow edema related to SIFs,31 which can be seen as early as 18 days after symptoms develop.28 Most cases demonstrate both marrow edema and a fracture line.35 Marrow edema is demonstrated as areas of increased signal intensity on T2-weighted and inversion-recovery images and low signal intensity on T1-weighted images.28,35,38 A hypointense fracture line is usually evident within the area of edema, though it is not seen in 7% of cases (Fig 7).35
MR images in different patients with SIFs. A, Coronal oblique T1-weighted image demonstrates patchy low-signal intensity edema and a hypointense fracture line. B and C, Coronal oblique inversion-recovery images demonstrate high-signal-intensity edema within the lateral sacrum. Note a horizontal component involving the sacral bodies in C.
Coronal oblique images in the plane of the sacrum better demonstrate the vertically oriented fractures and should be included in the imaging protocol if there is clinical suspicion (Fig 7).31 Unfortunately, most patients evaluated for back pain initially have undergone lumbar spine imaging, and coronal oblique imaging of the sacrum is not routinely performed. Again, radiologists should be aware of this pitfall when interpreting lumbar MR images of elderly patients.
MR imaging can usually differentiate marrow edema secondary to SIFs from malignancy, with fat-saturation and postgadolinium imaging being particularly useful in this regard.19 Occasionally, MR imaging can be confusing, especially if a fracture line is not evident, and correlative CT or follow-up imaging may be useful.
Treatment
Conservative Therapy
Conservative management of SIFs has been the standard of care, though suggested treatment regimens are quite variable. Some authors recommend strict bed rest and pain control,2,3,10 whereas others suggest moderation of activity supplemented with crutches or a walker in addition to analgesics.16 There have also been reports promoting early physical rehabilitation.20,23 Although most patients improve symptomatically following conservative therapy, the time course can be prolonged and quite variable. Symptoms resolve in most patients by 12 months but can vary between 6 and 15 months.2,3,15,20,23
However, not all patients improve with conservative therapy, and prolonged bed rest is associated with significant morbidity, especially in the elderly. Of primary concern is thromboembolic disease, with a reported incidence of deep venous thrombosis following pelvic fracture between 29% and 61%, and a 2%–12% incidence of pulmonary embolism.23 Other adverse sequelae associated with prolonged bed rest include loss of muscle mass, cardiac dysfunction, pneumonia, decubitus ulceration, and bone demineralization.23 In fact, half of patients with pelvic insufficiency fractures will not return to their prior functional level, and there is a reported 14.3% overall mortality.39
Sacroplasty
Sacroplasty has emerged as a minimally invasive alternative to conservative therapy for SIFs. Similar to vertebroplasty in the thoracolumbar spine, it involves injection of PMMA cement into the fractured sacrum under imaging guidance.5,6,40–42 The goal of sacroplasty is to provide early symptomatic relief, allowing more rapid mobilization. This would limit the need for significant narcotic analgesics, and lessen the risks associated with prolonged bed rest. Several retrospective and prospective case series suggest that sacroplasty can safely and effectively provide early symptomatic relief,5–7,41 though these findings have not been verified in controlled prospective randomized trials.
Injection of cement into the sacrum was first used for the therapy of painful bony metastases43,44 and was first described in sacral osteoporotic insufficiency fractures by Garant in 2002.40 Sacroplasty has increasingly been used for both these indications, though it has not yet achieved widespread acceptance, unlike vertebroplasty, probably due to the lack of validating controlled studies or because of unique technical considerations related to sacral anatomy.
The technical aspects of sacroplasty vary significantly between operators, including the technique of imaging guidance, as well as the needle approach. There are 2 basic needle approaches described in the literature: a posterior approach5,40–42,45 and the long-axis approach.36,46,47 A midline approach has also been used by some physicians to treat the horizontal or sacral body (zone 3) component of the fracture (F.R. Hellinger, unpublished data, 2009). Although the exact technique of these different approaches is beyond the scope of this article, a basic overview of the methodology and advantages of each is described.
Posterior Approach
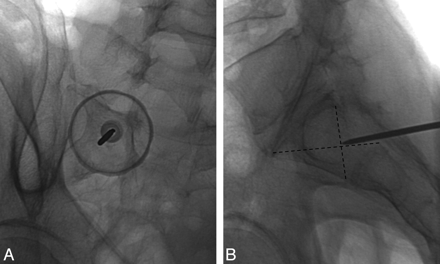
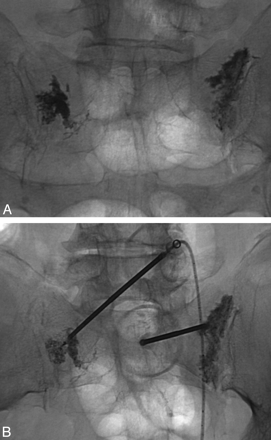
The posterior or dorsal approach is similar to that in used in vertebroplasty, and many authors probably use this approach, given their familiarity with vertebroplasty. The patient is placed prone on the procedure table. Following the administration of a local anesthetic, an 11- or 13-gauge bone biopsy needle is percutaneously inserted through the posterior sacral cortex into the sacral ala and lateral aspect of the sacrum (zone 1) in a plane parallel to the sacroiliac joint (Figs 8 and 9).5,40 Often, 2–3 needles are inserted on a single side to ensure adequate dispersal of cement throughout the sacrum. On a fluoroscopic anteroposterior projection, the image intensifier is lined up parallel to the L5-S1 disk space and the ipsilateral sacroiliac joint.48 On the lateral fluoroscopic view, the ventral cortical margin can be difficult to discern because of the unique shape of the sacrum with its concave ventral surface.5 Fluoroscopic landmarks have been confirmed with anatomic dissection and identify a well-defined triangular breach zone.49 Recently, a lateral target point for fluoroscopically guided sacroplasty has been described at the intersection of lines drawn through the corners of the S1 vertebral body (Fig 8).50 In our experience, acquiring a preoperative CT scan of the pelvis with multiplanar reformatted imaging is helpful to delineate the complex sacral anatomy for each patient.
Prone oblique and lateral radiographs during left S1 sacroplasty by using the posterior approach demonstrate appropriate needle tip placement at the target point defined by Jayaraman et al.50
Postprocedural (A) and intraoperative (B) prone radiographs from posterior-approach sacroplasty demonstrate deposition of cement into the sacral ala.
One of the concerns with fluoroscopically guided sacroplasty is inadvertent transgression of the ventral sacral cortex by the procedure needle. Venography through the procedure needle has been suggested as a method to confirm needle tip placement,40 though other authors have found limited success by using this technique.5 CT guidance allows accurate needle placement and allows the physician to remain confident that the needle tip remains intramedullary. Both CT5,47,51 and CT fluoroscopically41 guided sacroplasties have been described. However, observation of cement injection under fluoroscopy, either by using a mobile C-arm or transfer of the patient to a fluoroscopy suite, is recommended because it is difficult to monitor cement extrusion on CT.41 This is especially true in the craniocaudal direction, which is the preferred direction of cement migration given the commonly vertically oriented sacral fractures.
Long-Axis Approach
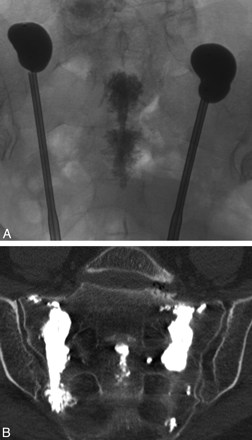
The long-axis approach to sacroplasty involves placement of needles from caudal to cranial along the longitudinal extent of the sacrum.36,46,47 The patient is placed prone, and the image intensifier or CT gantry is tilted to align with the plane of the sacrum more closely. This technique offers 2 theoretic advantages over the posterior approach. First, placement of the procedure needles along the longitudinal extent of the sacrum removes the risk of inadvertent penetration of the ventral sacral cortex. Additionally, cement can be extruded from the procedure needle more evenly along the vertically oriented sacral fractures, providing a more even distribution of cement rather than numerous relatively localized collections (Fig 10). Localized cement injections from the posterior approach also have a theoretically increased risk of early cement extravasation necessitating the delivery of smaller aliquots. Although the long-axis approach might intuitively appear advantageous, to our knowledge, the 2 techniques have not been compared in randomized controlled trials for safety and efficacy. To date, case series suggest that both techniques result in early symptomatic relief, with no significant reported complication.5,36,40,46
Intraoperative radiograph (A) and postprocedural coronal oblique CT scan (B) from long-axis-approach sacral kyphoplasty demonstrate more even deposition of PMMA cement along the lateral aspect of the sacrum. Note the cement within the sacral bodies from midline-approach sacroplasty, performed immediately prior. Courtesy of Frank R. Hellinger, Orlando General Hospital, Orlando, Florida.
Midline Approach
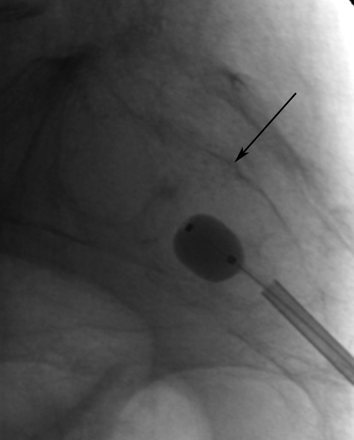
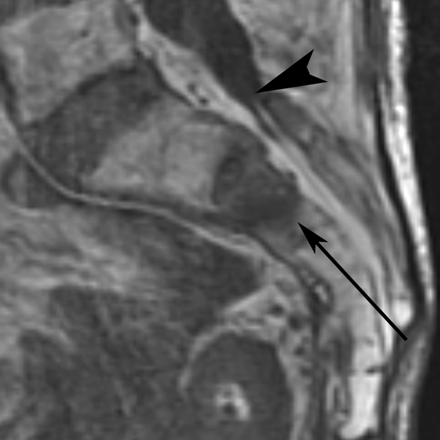
Although the techniques described above address the fractures involving the sacral ala, a midline approach has been used by some physicians to treat the horizontal component of SIFs when present (F.R. Hellinger, unpublished data, 2009). With the patient prone, an 11-gauge needle is placed under fluoroscopic guidance from caudal to cranial in the midline parallel to the plane of the sacrum (Fig 11). The needle transgressesthe central sacral canal and necessitates a preprocedural MR imaging to verify that the expected needle tract is below the caudal extent of the thecal sac (Fig 12). One operator has performed 37 midline sacroplasties with no significant or permanent complications (F.R. Hellinger, unpublished data, 2009).
Lateral radiograph during inflation of a 10-mm balloon in an S2 midline sacral body fracture (same patient as in Fig 12). Note that the dorsal cortical margin of the sacral body is well seen (black arrow). Courtesy of Frank R. Hellinger, Orlando General Hospital, Orlando, Florida.
T1-weighted sagittal MR imaging of the sacrum demonstrates fracture-related edema in the S2 sacral body. Note that the thecal sac ends at the S2 level (black arrowhead), well above the expected needle path for the midline approach (thin black arrow). Note prior L5 and S1 laminectomies. Courtesy of Frank R. Hellinger, Orlando General Hospital, Orlando, Florida.
Safety and Efficacy
In multiple case series36,40–42,45–47 and a prospective multicenter trial,6 sacroplasty has provided early subjective symptomatic relief in patients treated for SIFs. The prospective study of 52 patients treated with sacroplasty found a 50% reduction in the VAS at 2 days, 80% at 2 weeks, and 90% at 1 year.6 Additionally, the authors found that reduction in the VAS paralleled the reduction in the use of narcotic medication.6 Other retrospective case series corroborate these results, reporting long-term pain relief following sacroplasty for as long as 1.5 years after the procedure.8 In addition, patients reported improved ability to perform activities of daily living.8 Although these results are promising, they have not been prospectively compared with those of a control group of patients treated with conservative therapy.
These results are similar to our experience. Between 2003 and 2008, 26 patients were treated at Wake Forest University Baptist Medical Center with sacroplasty. Early symptomatic pain relief was noted in almost all patients without any significant complications, and results have been published.5,8
In addition to osteoporotic fractures, sacroplasty has been used to treat painful metastatic lesions in the sacrum.43,44 Case reports describe sacral cement injection for hepatocellular carcinoma,52 hemangioma,53 lung cancer and lymphoma,54 and renal cell carcinoma and myeloma.55
Complications of sacroplasty involve the unexpected extrusion of PMMA cement outside of the fractured sacrum, with untoward neurologic sequelae being the highest concern. Two cases of sacroplasty-associated cement leakage into the neural foramen have been described in the literature.6,42 In 1 case, there was a transient S1 neuritis, which resolved following transforaminal epidural steroid injection.6 Cement extrusion into the paraspinal soft tissues and sacroiliac joints can also be seen, though this is usually clinically insignificant unless it involves neural or vascular structures (Fig 13). Other theoretic complications of sacroplasty include extrusion of cement into the sacral spinal canal with associated neurologic compromise, infection, or venous emboli; however, to our knowledge, these have yet to be reported in the literature.
Axial CT scan following bilateral sacroplasty demonstrates leakage of PMMA cement into several sacral neural foramina and the left sacroiliac joint. Despite the extravasation, the patient was asymptomatic.
One technical consideration unique to sacroplasty involves the lack of resistance during cement extrusion. Unlike vertebral bodies during vertebroplasty, the capacious medullary cavity of the sacrum offers little resistance or feedback to the operator during cement extrusion.5,56 Close fluoroscopic monitoring is necessary to ensure that cement does not leak into the neural foramina, presacral space, or sacroiliac joint.
Other Treatment Options
Sacroplasty with balloon augmentation, or sacral kyphoplasty, has been described in 2 case reports suggesting the feasibility of this method.53,56 The sacral kyphoplasties were performed for osteoporotic fractures56 and a sacral hemangioma.53 Sacral kyphoplasty is technically similar to sacroplasty, and several balloon systems are commercially available. One suggested advantage of sacral kyphoplasty is the creation of a compacted bony layer outside the balloon, which could theoretically lower the likelihood of cement extravasation.56 One study evaluating balloon sacroplasty in cadaveric sacral models demonstrated more controlled localized deposition of cement.49
Fluoroscopic placement of transiliosacral screws has also been reported in case series as an alternative method of sacral stabilization in patients with SIF.57 A similar procedure using computer assistance for screw placement has also been reported.58
Biomechanics
Despite the subjective symptomatic relief experienced by patients, the exact mechanism of pain relief has not been fully elucidated. It has been suggested that excessive sheer strain on the sacral ala in insufficient bone results in fracture, with pain the result of micromotion at the fracture site.7 Biomechanical evaluation of cadaveric sacral models following sacroplasty has not shown any significant restoration of strength or stiffness following sacroplasty.16 Furthermore, the amount and location of PMMA cement used in sacroplasty has no bearing on the strength or stiffness restoration in cadaveric sacral models.59 However, these models assess the biomechanical properties of the entire sacrum and do not assess local biomechanical properties, especially near the cement-bone interface.
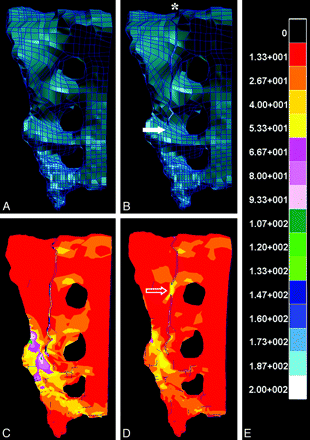
It has been suggested that sacral fracture stabilization and attenuation of fracture micromotion by the PMMA cement may underlie reports of sacroplasty-associated pain relief. FEA is an engineering method used to evaluate the mechanical properties of bone in computer-generated 3D geometric models of osseous structures. FEA has demonstrated an 83% decrease in maximal principal stress at the point of sacral fracture propagation in cadaveric sacral models after sacroplasty. FEA also demonstrated significantly diminished fracture gap micromotion after sacroplasty (Fig 14).48 Local compressive, tensile, and shear strains are also reduced 40%–60% following cement injection in nonfractured models, as documented by FEA.60 These biomechanical features may explain subjective pain relief following sacroplasty.
A and B, Intact (A) and fractured (B) finite-element models of the hemisacrum constructed from cadaveric CT data, with the asterisk and solid arrow (B) indicating fracture origin and point of fracture propagation, respectively. C–E, FEA reveals kilopascals of stress experienced by the hemisacrum after application of a 35-kg load according to the calibration scale (E), both before (C) and after (D) simulated sacroplasty at a point along the fracture (open arrow, D). Note that the point of fracture fusion (open arrow, D) subsumes a portion of the stress generated by the 35-kg load and may explain the attenuated stress surrounding the site of fracture propagation compared with the presacroplasty model (C).
Sacroplasty has been criticized for disrupting normal osseous healing following sacral fracture.61,62 Tsiridis62 noted that most patients with SIF recover completely with conservative therapy, albeit along an often prolonged and variable time course. Ehara61 suggested that sacroplasty may be more indicated in patients with delayed union or prolonged severe pain.
Conclusions
Sacral fractures are a common yet underdiagnosed cause of low back pain, predominantly in elderly women with osteoporosis. Plain radiographic, scintigraphic, CT, and MR imaging findings have been well described in the literature. Bone scintigraphy and MR imaging are the most sensitive examinations and can be diagnostic, especially in the correct clinical setting. CT can be a helpful adjunct in cases in which scintigraphy and MR imaging are inconclusive.
Sacroplasty has recently emerged as a minimally invasive alternative to conservative treatment for SIFs. Prospective studies and case reports suggest that it is a safe and effective therapy, resulting in early symptomatic relief in patients with these fractures. However, randomized controlled trials comparing sacroplasty and conservative therapy are necessary to validate this technique.
The optimal technique for performing sacroplasty remains uncertain because various combinations of imaging guidance have been used and several different needle approaches have been described. Additionally, sacral kyphoplasty has been described. At this point in time, the exact technique used is dependent on the experience and comfort of the physician because all reported techniques have similar subjective pain relief and no significant reported complications. Still, studies comparing the safety and efficacy of the various techniques are necessary.
Acknowledgments
We thank Frank R. Hellinger, MD, PhD, Orlando General Hospital, Orlando, Florida, for technical advice regarding midline sacroplasty.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- Copyright © American Society of Neuroradiology