Abstract
BACKGROUND AND PURPOSE: The fate of contrast stasis within an aneurysmal sac after coiling has not been established. We followed and evaluated the potential risks of recanalization of unruptured aneurysms embolized with BPCs for 2 years.
MATERIALS AND METHODS: A total of 301 unruptured aneurysms in 252 patients were treated with BPCs. Contrast stasis was observed on initial postembolization angiograms in 104 (34.6%) of these aneurysms. For follow-up, skull images by an angiographic unit (at 3, 9, 15, and 21 months), CE-MRA including TOF source images (at 6, 12, and 18 months), and DSA (at 24 months) were used.
RESULTS: In 89 (85.6%) of the 104 aneurysms with contrast stasis, the stasis disappeared on 6-month MRAs and occlusions remained unchanged without recanalization for 2 years. In the remaining 15 (14.4%), recanalization occurred during follow-up. The presence of contrast stasis was not found to be associated with the obliteration rate (P = .641) or packing attenuation (aneurysms without contrast stasis 30.7% ± 11.18 versus aneurysms with contrast stasis 33.0% ± 12.11, P = .113). Contrast stasis was not found to be a risk factor for recanalization (15/104 [14.4%] versus 29/197 [14.7%], P = 1.000).
CONCLUSIONS: Contrast stasis is a benign angiographic finding that can disappear within 6 months on follow-up MRA. In addition, contrast stasis was not found to be associated with a low obliteration rate or packing attenuation or to be a risk factor for recanalization. The present study shows that aneurysms with contrast stasis on initial postembolization angiograms are no more likely to recanalize than aneurysms without contrast stasis.
Abbreviations
- BPC
- bare platinum coil
- CE
- contrast enhanced
- DSA
- digital subtraction angiography
- MIP
- maximum intensity projection
- MRA
- MR angiography
- TOF
- time of flight
Complete obliteration is the primary goal of intracranial aneurysm coiling. Endovascular surgeons make every effort to achieve this goal, but forceful final coil insertion to achieve complete angiographic results is not always safe. Therefore, the potential risks and benefits of aggressive coil packing should be weighed carefully. In our practice, we have often finished the procedure for unruptured aneurysms, despite contrast filling within the coil mass or sac, when contrast filling persisted until the venous phase by angiography and the coil mass shape seemed satisfactory. Of course, these aneurysms with contrast stasis are not classified as complete occlusion or perfect angiographic results, but as “residual filling of aneurysm dome.” We questioned whether this treatment policy is justified because contrast stasis could undergo either subsequent thrombosis with involution,1,2 or recanalization.2 This study summarizes 2-year follow-up results of 104 aneurysms that showed contrast stasis within the aneurysmal sac after coiling.
Materials and Methods
Patient Selection
We reviewed the clinical and radiologic data of patients with unruptured aneurysms who underwent elective endosaccular coil embolization between May 2003 and January 2008 at our institution. Ruptured aneurysms were not included because the thrombogenic environment could have affected results, and for the same reason, unruptured aneurysms coiled at the same time as ruptured aneurysms were excluded. In addition, patients not followed by regular imaging during the 2-year follow-up after coil embolization were also excluded. A total of 301 aneurysms in 253 patients were included.
Coil Embolization Procedure
All aneurysm coiling was performed with the patient under general anesthesia by using a biplane angiographic unit, Integris Allura (Philips Healthcare, Best, the Netherlands). Technical details of the aneurysm coiling were conventional and have been previously described in detail.3 All aneurysm embolizations were performed by using detachable platinum coils, including Guglielmi (Boston Scientific, Fremont, California), MicroPlex (MicroVention, Aliso Viejo, California), Trufill-DCS (Cordis, Miami Lakes, Florida), and Axium (ev3, Irvine, California) coils. Modified coils, such as Matrix (Boston Scientific) and HydroCoil (MicroVention), were not used. Final postembolization angiography was performed at the working projection to detect any residual contrast filling, thrombus formation, or parent artery compromise. Frontal and lateral projections were also acquired at the end of the procedures. In all cases, we used iohexol (300 mg I/mL, Omnipaque 300; GE Healthcare, Milwaukee, Wisconsin) as contrast and obtained final angiographic images by using an injector manufactured by Liebel-Flasheim (Angiomat Illumena; Cincinnati, Ohio). The usual contrast-injection rate and total volume, 3 mL/s and 5 mL, respectively, were used for the internal carotid and vertebral arteries. In all cases, systemic heparinization was performed after placing a femoral introducer sheath. In line with our embolization protocol, 3000 IU of heparin was administered as an intravenous bolus injection, and this was followed by an additional 1000 IU per hour. Heparin was discontinued after embolization in all patients.
Aneurysmal and Procedure-Related Factors
“Contrast stasis” was defined as visual contrast filling within the coil mass or any part of the aneurysm fundus persisting to the venous phase on postembolization angiograms (operative, frontal, or lateral projection).1 Sizes of contrast stasis were calculated by using the following formula:

This area ratio was measured by using PACS software with its freehand region-of-interest measurement tools. Depending on size, contrast stasis was divided into 3 categories: small (≤5%), medium (5%–10%), and large (≥10%). Locations of contrast stasis were classified as proximal (near the aneurysmal neck), central, and peripheral (near the dome).
The following aneurysmal factors were recorded and used in the analysis: aneurysm location (Table 1), type (sidewall, bifurcation), size (small, ≤5 mm; medium, 5–15 mm; large, ≥15 mm), neck size (≤4 mm, >4 mm), dome-to-neck ratio, and volume (cubic centimeters). Procedure-related factors, including embolization methods (catheter only, balloon assisted, stent-assisted), aneurysm obliteration grade (0, complete; 1, occlusion ≥90%; 2, occlusion 70%–89%; 3, occlusion 50%–69%; 4, occlusion 25%–49%; 5, occlusion <25%), and packing attenuation (percentage), were measured and analyzed.
Aneurysm location patterns and prevalence of contrast stasis
The following formulae were used to calculate aneurysm volumes and packing densities:
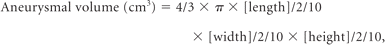
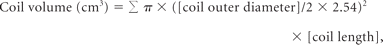
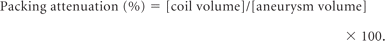
Imaging Follow-Up after Coil Embolization
The tools used during follow-up were skull imaging with an angiographic unit (working, conventional frontal, and lateral projections), CE-MRA (including TOF source images), and DSA. Skull radiographs were obtained at 3, 9, 15, and 21 months and MRA at 6, 12, and 18 months postembolization. DSA was performed at 24 months postembolization. In some cases (91, 30.2%) treated during 2003–2005, DSA was performed at 12 months after embolization instead of MRA, in accord with the follow-up protocol used at that time. Whenever findings were obtained that suggested recanalization on skull images or MRA, DSA was also performed.
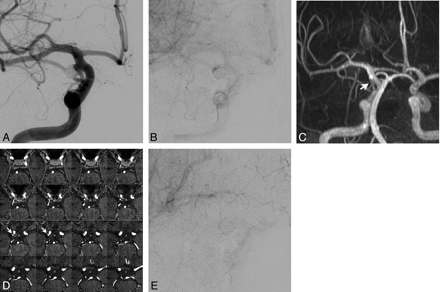
Right posterior communicating artery aneurysm with contrast stasis. A, This initial angiogram obtained immediately after coiling in a working projection during the arterial phase reveals grade 1 obliteration (packing attenuation, 30.3%) with a small neck remnant. B, This venous phase image shows medium-sized (6.8%) contrast stasis near the aneurysmal dome. C and D, Six-month follow-up CE-MRA and CE-TOF source images show the neck remnant (arrow), but no inflow into the coiled aneurysm. E, This angiogram obtained in the same working projection during the venous phase 2 years after coil embolization shows no contrast stasis.
MRA was conducted by using a 1.5T or 3T scanner (1.5T, Intera; 3T, Achieva; Philips Healthcare) by using a sensitivity encoding phased-array head coil. The MR imaging protocol included axial T2-weighted imaging and CE-TOF MRA. The parameters used for CE-TOF MRA were the following: FOV, 200 × 200 mm; TR/TE, 25/3.45 ms; flip angle, 20°; section thickness, 1.0 mm (gap, 0.5 mm); and acquisition time, 8 minutes. TOF MRA data were postprocessed on a workstation (ViewForum; Philips Healthcare) and reconstructed into MIP images. A bolus of 0.2 mL/kg of gadodiamide at a concentration of 0.5 mmol/mL (Omniscan; GE Healthcare) was injected at 2 mL/s with a 15-mL saline flush by using a power injector. Angiography started automatically with bolus detection.
Follow-Up of Contrast Stasis and Evaluation of Recanalization
Contrast stasis was re-evaluated by follow-up MRA or DSA and classified as disappeared, improved (decreased in the size of contrast stasis), stable (size unchanged), and aggravated (increase in size). “Recanalization” was defined as aneurysm recurrence evident by neck growing, coil compaction, coil degradation, or new sac formation. In addition, newly visualized contrast filling inside an aneurysm was also considered to indicate recanalization.
All radiologic data were reviewed independently by 2 neuroradiologists (C.J. and M.H.H.) who were unaware of clinical results.
Statistical Analyses
Statistical analysis was conducted by using the Statistical Package for the Social Sciences, Version 17 (SPSS, Chicago, Illinois). The Student t test was used to evaluate univariate associations between contrast stasis and numeric factors. The χ2 or Fisher exact test was used to evaluate relationships between contrast stasis and nominal factors. Statistical significance was accepted for P values < .05.
Anterior communicating artery aneurysm with contrast stasis. A, Arterial phase angiogram obtained immediately after coil embolization in a working projection reveals grade 2 obliteration (packing attenuation, 24.5%) with a neck remnant and no contrast filling in the coil mass. B, Initial angiogram in a lateral projection during the venous phase shows a small (4.1%) amount of contrast stasis. C and D. Six-month follow-up CE-MRA and CE-TOF source images show the neck remnant (arrow), but no inflow into the coil mass. E, Venous phase lateral-projection angiogram at the 2-year follow-up shows no contrast stasis.
Results
Of the 301 aneurysms enrolled in this study, contrast stasis was observed on initial postembolization angiograms in 104 (34.6%, Table 2). In terms of the contrast-stasis size, 24 aneurysms were small (23.1%), 49 were medium (47.1%), and 31 were large (29.8%, Table 3). Seventy-six (73.1%) had a contrast stasis located near the dome (peripheral type). There were 12 (11.5%) proximal types and 16 (15.5%) central types based on location of contrast stasis. In 89 (85.6%) of the 104 aneurysms with contrast stasis, the coil mass maintained its shape during 2 years of follow-up. In these 89 cases, contrast stasis disappeared on 6-month MRAs, and this disappearance was maintained on 12-month MRA or DSA, 18-month MRA, and 2-year follow-up DSA images, regardless of contrast-stasis size or location. In the remaining 15 (14.4%) aneurysms with contrast stasis, recanalization occurred during follow-up. However, the existence of contrast stasis on final angiograms was not found to be significantly associated with the future development of recanalization (29 [14.7%] of 197 aneurysms without contrast stasis developed recanalization, P = 1.000).
The basic characteristics of the 2 study groups according to the occurrence of contrast stasis
Incidence of recanalization and retreatment according to the presence of contrast stasis
No significance was found between the size of contrast stasis and recanalization (P = .930). In terms of contrast stasis location, a proximal location of contrast stasis near the aneurysmal neck showed a higher incidence of recanalization than other locations, but this was not significant (P = .540). Of the recanalization types, coil compaction was more frequent than neck growth for aneurysms with contrast stasis. However, the existence of contrast stasis was not found to be significantly associated with recanalization type (P = .320). Of 44 aneurysms that showed recanalization, retreatment was performed in 11 (25%; group without contrast stasis, 7/29 [24.1%] versus group with contrast stasis, 4/15 [26.7%], P = 1.000). No aneurysms bled during the follow-up period.
The incidence of contrast stasis was not found to be significantly associated with obliteration grade (P = .641, Table 2). Aneurysms showing contrast stasis had a slightly lower packing attenuation (30.7% ± 11.18) than those not showing contrast stasis (33.0% ± 12.11), but again, this was without statistical significance (P = .113). Univariate analysis showed that a sidewall−type aneurysm (P < .001), a larger diameter aneurysm (P < .001), a larger neck size (P < .001), a larger aneurysmal volume (P = .035), and balloon- or stent-assisted embolization (P = .001) were significantly associated with the occurrence of contrast stasis.
Discussion
Angiographic contrast filling means that an aneurysm has not been completely occluded. Contrast is visualized but rapidly disappears with blood flow if space between coils is large enough to allow active flow. Sometimes, contrast remains longer; this finding indicates that blood flow is restricted, and in such cases, contrast can be visualized until the venous phase of an angiographic run or later. In this article, we call this restricted-flow situation “contrast stasis.” In practice, efforts to pack the last coils have often produced undesirable complications, such as, thrombus formation, thrombus migration, coil stretching, and others. Occasionally, endovascular surgeons are faced with making a decision to finish the procedure despite visual contrast filling or to proceed. In our practice, we have often chosen to finish the procedure in such situations, if contrast filling persists until the venous phase, especially in unruptured cases. However, we have been very curious about the fate of cases showing contrast stasis.
To the best our knowledge, only 1 previous report has addressed the fate of contrast stasis,1 but this previous study was conducted to evaluate the efficacy of HydroCoil embolization. Accordingly, to our knowledge, the present study is the first to reveal the prognosis of contrast stasis for aneurysms treated with BPCs.
In the present study, the following findings were notable: First, contrast stasis was obliterated spontaneously. All cases with contrast stasis on initial angiography performed postembolization had resolved by follow-up MRA at 6 months postembolization. Furthermore, this finding did not change on serial follow-up during 2 years if recanalization did not occur. Second, aneurysms with contrast stasis on initial angiography did not show higher recanalization rates. Finally, contrast stasis was not found to imply a low obliteration rate or packing attenuation. Contrast stasis indicates that blood is entrapped inside coil frames and that the coil architecture does not permit free blood flow. This coil architecture within aneurysmal sacs is affected by various aneurysmal and procedural factors and by variables related to the occurrence of contrast stasis, such as aneurysmal type, diameter, volume, neck size, and the embolization method used, as was found in the present study. Therefore, coil architecture is case-dependent, and contrast stasis develops in some cases, even though coiled aneurysms have the same obliteration rate or packing attenuation.
The main reason for treating unruptured aneurysms is to prevent future rupture, and complete aneurysm packing is known to be important in this context.2,4–7 However, our study shows that contrast filling inside aneurysms, if it persists to a late stage, does not necessarily indicate incomplete aneurysm packing and, thus, risk recurrence, and that most of the contrast stasis, in such cases, will spontaneously resolve. This finding implies that we do not need to take the risks associated with inserting additional coils to occlude the affected aneurysmal region.
The present study has several limitations. First, it is limited by its retrospective nature and by the 11 cases with contrast stasis that did not undergo follow-up. Second, MRA was used for regular follow-up, though we performed DSA at 2-year follow-ups in all patients enrolled. MRA and DSA findings have been shown to be well-correlated,8–11 and MRA may be more sensitive to residual flow in aneurysms than DSA, which may be affected by the opacity of the coils.12,13 However, DSA is still the procedure of choice for the follow-up of contrast stasis; thus, our results should be confirmed by DSA studies.
Conclusions
Contrast stasis is a transient angiographic finding, which disappears within 6 months after coil embolization on follow-up MRA. Aneurysms with contrast stasis on initial angiograms obtained immediately after coil embolization were found to have a recanalization rate similar to that of aneurysms without contrast stasis. Our study shows that the risks taken to insert additional coils to occlude aneurysms with contrast stasis may be unnecessary.
Footnotes
-
This work was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (grant A06–0171-B51004–06N1–00040B).
Indicates open access to non-subscribers at www.ajnr.org
References
- Received April 5, 2010.
- Accepted after revision May 13, 2010.
- Copyright © American Society of Neuroradiology