Abstract
BACKGROUND AND PURPOSE: DIBSGs have the worst prognosis among pediatric brain tumors with no improvement of outcome for several decades. In this study, we determined whether diffusion imaging could improve patient stratification and our understanding of the impact of therapies.
MATERIALS AND METHODS: Nine baseline and 24 follow-up DTI studies performed in 9 patients on a 1.5T clinical MR imaging scanner were reviewed. ADC and FA were measured for the whole lesion and at 5 anatomic levels: the rostral medulla, caudal pons, midpons, rostral pons, and caudal midbrain. Reference data were obtained from 8 controls with normal brain stem, 6 patients with medulloblastoma, and 7 patients with pilocytic astrocytoma.
RESULTS: ADC was higher in untreated DIBSG than in normal brain stem and medulloblastoma (1.14 ± 0.18 [×10−3 mm2/s] versus 0.75 ± 0.06 and 0.56 ± 0.05, both P < .001). FA was lower in DIBSG than in normal brain stem (0.24 ± 0.04 versus 0.43 ± 0.02, P < .001) but was higher than that in pilocytic astrocytoma (0.17 ± 0.05, P < .05). Lower baseline ADC and higher FA correlated with a worse clinical course. Correlations were more significant at the caudal midbrain than in other regions. ADC decreased and FA increased after RT. Changes of FA after RT at the caudal midbrain correlated with event-free survival.
CONCLUSIONS: Baseline ADC and FA of DIBSG revealed hypocellular tumors with extensive edema. Diffusion changes after therapy implied reduced edema but did not support a significant response to therapy. The significance of diffusion properties varied with anatomic locations, the caudal midbrain being particularly important.
Abbreviations
- ADC
- apparent diffusion coefficient
- ADCCM
- ADC at the caudal midbrain
- ADCLesion
- mean ADC of whole lesion calculated from the 5 anatomic regions examined
- ADCmax
- largest ADC value measured in the 5 anatomic regions examined
- ADCmin
- lowest ADC value measured in the 5 anatomic regions examined
- ADCMP
- ADC at the mid pons
- ADCRP
- ADC at the rostral pons
- AVA
- Avastin (bevacizumab)
- Bsl
- baseline
- CP
- carboplatin
- ΔADC
- change of ADC between the baseline study and the study immediately after completion of RT
- ΔFA
- change of FA between baseline study and study immediately after completion of RT
- ΔTEFS
- event-free survival after therapy
- ΔTSurvival
- survival time after diagnosis
- DIBSG
- diffuse intrinsic brain stem glioma
- DTI
- diffusion tensor imaging
- ETO
- etoposide
- FA
- fractional anisotropy
- FACM
- FA at the caudal midbrain
- FALesion
- mean FA of the whole lesion calculated from the 5 anatomic regions examined
- FAmax
- largest FA value measured in the 5 anatomic regions examined
- FAmin
- lowest FA value measured in the 5 anatomic regions examined
- GT
- gadolinium-texaphyrin
- IRI
- irinotecan (Camptosar)
- n.d.
- not determined
- RT
- radiation therapy
- Early post-RT
- within 3 months of completion of RT
- Late post-RT
- >3 months after completion of RT
- TEM
- temozolomide (Temodar)
- WHO
- World Health Organization
DIBSG accounts for approximately 15% of childhood central nervous system tumors1–3 and 80% of all brain stem gliomas.4 These tumors are inoperable and highly resistant to radiation therapy and chemotherapy. Because biopsies are not generally performed, the biology and status of these tumors in individual subjects are unknown and physicians have limited means for patient stratification and timely evaluation of the effectiveness of their therapies.
MR imaging is considered the criterion standard for diagnosing DIBSG. However, standard features such as lesion size or contrast accumulation at presentation generally do not correlate with disease course.5–8 On the other hand, it has been reported that diffusion MR imaging provides information that complements conventional pre- and postcontrast anatomic imaging in gliomas in adults.9–13 Specifically, the ADC has been reported to be a useful clinical prognostic biomarker for survival in patients with malignant supratentorial astrocytoma14–16 and a more sensitive indicator for response to therapy than conventional imaging techniques.15,16
DTI is a variant of diffusion imaging by which the directionality of the diffusion of water molecules can be probed. A readily obtained parameter is the FA, which describes to what extent water diffusion occurs anisotropically (for example along the nerve fibers). In previous studies, it was reported that anisotropic diffusion in pontine tumors was significantly lower than that in control tissue. This was attributed to tumor infiltration and the destruction of the highly directional white matter fiber tracts.17–19 The goals of our study were to evaluate whether ADC and FA measurements before therapy and after treatment can improve initial patient stratification, assess the response to therapy, and improve our understanding of the impact of current therapies.
Materials and Methods
Patients and Control Subjects
ADC and FA maps obtained from 9 patients with DIBSG were evaluated. All patients had a baseline DTI study before treatment and at least 1 follow-up study after therapy, and a total of 33 DTI studies were analyzed. Diagnoses of DIBSG were established on the basis of the initial MR imaging findings and the clinical course typical for this disease. One patient underwent a pretreatment biopsy at a different hospital immediately before being referred to this institution for therapy. The lesion in this patient also underwent postmortem examination at the time of death. No other biopsies or postmortem examinations were performed for the remaining subjects. The medical records of all patients were reviewed to determine ΔTSurvival and ΔTEFS (Table 1).
Demographics of patients with DIBSG included in this study
“Closest-to-normal” control data were obtained from 8 age-matched subjects with no brain stem pathology (3 girls, 5 boys; 8.0 ± 2.7 years of age). These subjects had unrelated indications for MR imaging, which included headaches, seizures, and ruling out tumors (negative), with all findings being interpreted as normal. In addition, ADC and FA maps of 6 patients with medulloblastoma (3 girls, 3 boys; 7.2 ± 4.1 years of age) and 7 patients with pilocytic astrocytoma (2 girls, 5 boys; 5.5 ± 5.4 years of age) were analyzed to obtain reference data from other pediatric tumors. Subgroups of patients and controls were enrolled in prospective research projects approved by the institutional review board. Parental consent and assent of subjects older than 8 years of age were obtained. For the remaining subjects, the institutional review board waived the review of already existing data and the requirement to obtain consent.
Data Acquisition and Analysis
Acquisition.
All subjects were scanned by using a 1.5T MR imaging system (Signa LX; GE Healthcare, Milwaukee, Wisconsin). All diffusion measurements were integrated into the standard imaging protocol. An echo-planar sequence with a TE of 85 ms, 5- to 7-mm section thickness, 20- to 26-cm FOV, 128 × 128 matrix size, 25 directions, and a b-value of 1000 s/mm2 was used for all measurements. Software provided by the manufacturer was used to calculate ADC and FA maps. The maps were then stored and reviewed by using the hospital PACS system.
Analyses.
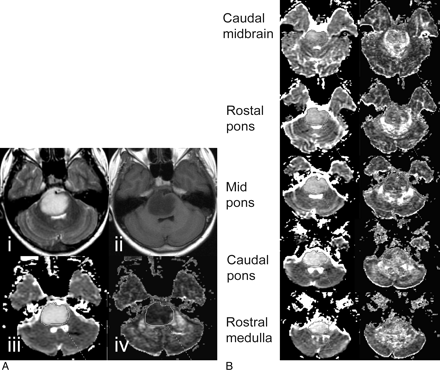
ADC and FA values of the brain stem gliomas were measured at 5 different axial levels of the brain stem: the caudal midbrain, rostral pons, mid pons, caudal pons, and rostral medulla (Fig 1). To ensure consistency and consensus, 1 author (H.J.C.) outlined the regions and at least 1 board-certified neuroradiologist (A.P. and/or M.D.N.) subsequently confirmed them. Areas with necrosis and cystic degeneration were excluded to the extent possible. Previous studies have shown the importance of measuring ADCmin of a lesion, which was set in this study to the lowest ADC measured in any of the 5 anatomic areas. We also analyzed the FAmin and FAmax obtained from the lowest and highest FA of any of the 5 regions, respectively, as well as the ADCmax. Also reviewed were ADCLesion and FALesion as well as the mean value for single anatomic regions (eg, ADCRP and FACM). ΔADC and ΔFA between baseline and an MR imaging study conducted immediately after completion of radiation were correlated with ΔTEFS after therapy.
A, Anatomic T2-weighted (i) and T1-weighted MR images (ii) of a DIBSG at initial presentation. Shown also are the ADC map (iii) and the FA map (iv). B, For the same patient, regions of interest for the different anatomic regions are drawn on ADC and FA maps obtained at a follow-up study 8.9 months after initial presentation.
Control data from subjects with normal MR imaging findings of the brain stem were obtained in the same fashion. ADC and FA values from medulloblastoma and pilocytic astrocytoma were obtained by marking solid areas of the tumors in representative images.
Statistics
ADC and FA values are reported in mean values and SDs. Unpaired 2-tailed Student t tests were used to determine significant differences between measurements in DIBSG and controls, medulloblastoma, and pilocytic astrocytoma. An analysis of variance test was used to determine the significance of variations of ADC and FA over different anatomic regions. Linear regression analysis was used to analyze the correlation between survival time and initial diffusion properties of the lesions. The time course of ADC and FA in this disease was determined at 3 time points: at baseline; at an early post-RT period, which included the scan immediately after completion of RT and the next follow-up scan within 3 months; and at a late post-RT period thereafter. The cutoff of 3 months after completion of therapy between the 2 post-RT periods was selected to ensure an approximately equal number of studies within each period to maximize statistical power. Linear regression analysis was also used to determine whether there was a correlation between ΔTEFS and changes of diffusion properties immediately after therapy. Reported P values were not corrected for multiple comparisons.
Results
Baseline ADC and FA in DIBSG versus Normal Brain Stem, Medulloblastoma, and Pilocytic Astrocytoma
The mean ADCLesion at baseline of DIBSG was 1.14 ± 0.18 (×10−3 mm2/s). This was significantly higher than the mean ADC of normal brain stem in controls obtained from comparable regions (0.75 ± 0.06, P < .001). Baseline ADCLesion of DIBSG was also significantly higher than mean ADC of cellular (WHO grade IV) medulloblastoma (0.56 ± 0.05, P < .001) but was comparable with that of hypocellular (WHO grade I) pilocytic astrocytoma (1.14 ± 0.27, not significant) (Table 2). Across different anatomic regions, the baseline ADCs in DIBSG were 1.19 ± 0.21 (rostral medulla), 1.24 ± 0.24 (caudal pons), 1.20 ± 0.19 (mid pons), 1.09 ± 0.16 (rostral pons), and 0.99 ± 0.18 (caudal midbrain). There was no statistically significant difference in baseline ADCs at different anatomic levels (analysis of variance).
ADC (×10−3 mm2/s) and FA measurements in patients with DIBSG at various stages of the disease, in controls, and in untreated medulloblastoma and pilocytic astrocytoma
The baseline FALesion of DIBSG was 0.24 ± 0.04 and was significantly lower than the mean FA of normal brain stem in the controls (0.43 ± 0.02, P < .001). Baseline FALesion of DIBSG was not significantly different from the mean FA of medulloblastoma (0.24 ± 0.10, not significant.). On the other hand, FALesion was significantly higher than the mean FA of pilocytic astrocytoma (0.17 ± 0.05, P < .05) (Table 2). Across different anatomic regions, the baseline FA values in DIBSG were 0.25 ± 0.05 (rostral medulla), 0.23 ± 0.06 (caudal pons), 0.22 ± 0.05 (mid pons), 0.23 ± 0.04 (rostral pons), and 0.29 ± 0.06 (caudal midbrain). FA values at different anatomic levels of the lesions were not significantly different.
Correlation of Baseline ADC and FA with ΔTSurvival
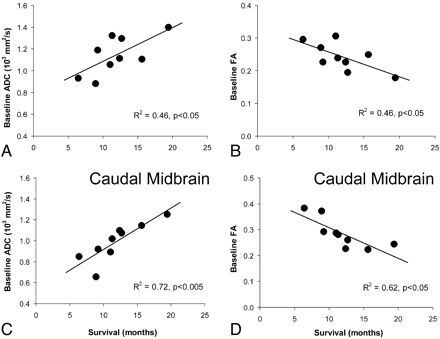
A significant positive linear correlation (R2 = 0.46, P < .05) between baseline ADCLesion and survival and a negative linear correlation (R2 = 0.46, P < .05) between FALesion and survival were noted (Fig 2A, -B). Also, ADCmin and FAmax correlated with outcomes (R2 = 0.54, P < .05 and R2 = 0.55, P < .05, respectively).
A positive linear correlation between ADC and survival (A) and a negative linear correlation between FA and survival (B) are noted. C and D, When individual anatomic regions are analyzed, ADCCM and FACM showed the most significant correlation (C and D).
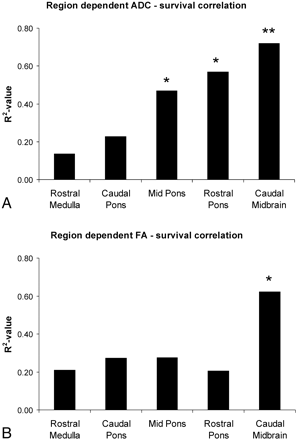
Of the 5 anatomic regions analyzed, ADCCM and FACM showed the most significant correlation with survival (ADCCM: R2 = 0.72, P < .005 and FACM: R2 = 0.62, P < .05) (Fig 2C, -D). ADCMP and ADCRP also correlated with survival, though to a lesser extent than at caudal midbrain (Fig 3A). FA measurements outside the caudal midbrain did not correlate with outcome (Fig 3B).
Shown are the R2 values of the linear correlation analyses between ADC (A) and FA (B) for the 5 anatomic regions. Correlation coefficients are highest and the most significant correlation is noted for the caudal midbrain. ADCMP and ADCRP also correlate with survival, albeit to a lesser extent than at the caudal midbrain. FA measurements outside the caudal midbrain do not correlate with outcome.
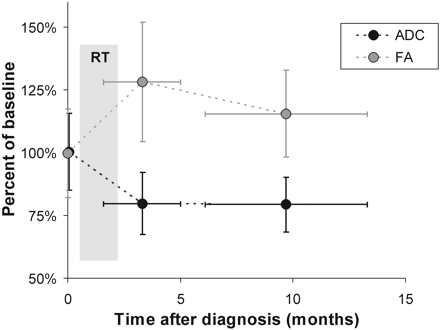
Time Courses of ADC and FA
All except 1 patient (subject 6) had a DTI study performed within 3 months after completion of radiation therapy (3.3 ± 1.7 months after initial diagnosis). ADCLesion at this early post-RT period was 0.91 ± 0.10 (×10−3 mm2/s), which was significantly different from the baseline ADCLesion (1.14 ± 0.18, P < .005). Thereafter, at the late post-RT period (9.7 ± 3.6 months after initial diagnosis), no significant change was observed (0.91 ± 0.10) (Table 2 and Fig 4). FALesion increased significantly from initially 0.24 ± 0.04 to 0.32 ± 0.08 (P < .05) during the early post-RT period. During the late post-RT period, FAmean was 0.29 ± 0.05, which was not significantly different from baseline (Table 2 and Fig 4).
ADC decrease and FA increase significantly between baseline and the early post-RT period. Thereafter no significant changes are observed.
Correlation of ΔTEFS with ΔADC and ΔFA
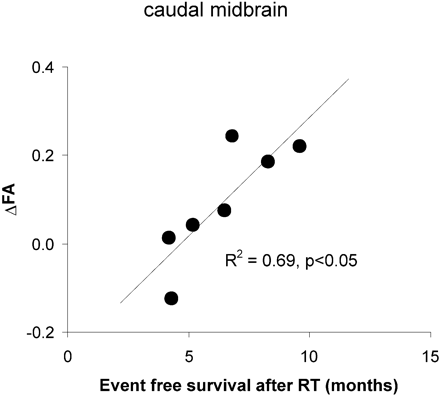
Seven subjects had DTI studies performed before and immediately after RT (within 2 weeks of completion of RT). No significant correlations between ΔADC and ΔTEFS were observed for whole lesion ADC. There were also no significant correlations between ΔADC and ΔTEFS in individual regions and for minimum or maximum ADC. A statistically significant positive correlation between ΔFA in the caudal midbrain and ΔTEFS (R2 = 0.69, P < .05) was noted (Fig 5). ΔFA at other anatomic levels did not correlate with ΔTEFS.
A statistically significant positive correlation between ΔFA in the caudal midbrain and ΔTEFS is noted.
Discussion
Most childhood brain stem gliomas are diffuse intrinsic tumors that involve the pons and other adjacent brain stem sites.2,20–22 These tumors are considered inoperable due to their location, regardless of their histology, and carry the worst prognosis of tumors in pediatric neuro-oncology. Conventional treatment for this disease is radiation therapy, which will result in a transient benefit for more than half of patients. To date, the addition of radiosensitizers, chemotherapy, or immunotherapy to radiation therapy has not demonstrated any improvement in survival.20,21,23,24 Because biopsies are usually not obtained, physicians know little about the pathologic status or the impact of therapy. Also, monitoring with standard MR imaging is frequently not useful. Initial features, such as lesion size and contrast accumulation as well as changes observed on MR images, often do not correlate with progression, whereas in some patients, obvious clinical deterioration was observed despite no changes on MR imaging.6,25 This lack of appropriate monitoring tools compromises patient stratification and slows down clinical research, and there has been no improvement in mean survival time during the past several decades. In an effort to improve this situation, the overall objective of this research was to evaluate whether diffusion MR imaging and DTI can provide much-needed quantitative biomarkers of disease status and response to therapy.
Diffusion Properties of DIBSG at Initial Presentation
ADC mapping has been shown to provide important prognostic information in malignant supratentorial astrocytoma in adults. Specifically, tumors with particularly low ADCmin values had the worst clinical courses.14,26 This observation is likely explained by the fact that increased tumor cellularity distinguishes grade IV glioblastoma from lower grade gliomas on pathology (among other features such as high vascularity and high mitotic index). Highly cellular tumors or areas of particularly high cellularity restrict diffusion, resulting in low ADC measurements. Consistent with these findings in adults with gliomas, patients with DIBSG with lower ADC at presentation generally did worse in this study. However, all DIBSGs are relatively hypocellular lesions. For example, the mean ADC of DIBSG was significantly higher (with no overlap) than the mean ADC of high-grade (WHO IV) hypercellular medulloblastoma. The diffusion in DIBSG was also significantly less restricted than that in normal brain stem.
Also observed was a negative correlation between initial FA and survival times, indicating that patients with tumors with higher baseline anisotropic water diffusion are at higher risk for rapid disease progression. In the normal brain stem, water diffusion is highly directional due to white matter tracts, and FA in our controls with normal brain stem was approximately twice as high as that in DIBSG. Our initial hypothesis was that a reduction of FA in DIBSG would correlate with the extent of tumor infiltration and destruction of anisotropic normal tissue as previously suggested17–19 and that lower FA would predict a poorer clinical course. However, our results clearly rejected this hypothesis. We observed a negative correlation (instead of a positive one) between initial FA and survival length, indicating that patients with tumors of higher baseline anisotropic water diffusion are at higher risk for rapid disease progression.
Changes of ADC and FA after Therapy
In adult gliomas, a posttreatment increase of ADC was a predictor of a more favorable clinical course.15,16,27–29 Therefore, the changes after RT hoped for by the treating clinicians—in terms of diffusion properties—are an increase of ADC (and a drop of FA). These changes would be consistent with increased extracellular space caused by cell death, which in turn might suggest a reduction of tumor volume before changes on conventional MR imaging.15,16,27–29 However, the opposite was observed in this study. There was a significant ≈20% reduction of ADC, whereas FA increased by ≈30% between baseline and the early posttreatment phase. With ADC and FA as surrogates for tissue status at the microscopic level, this finding would suggest that lesions after RT are apparently more cellular with less extracellular fluid accumulation. It is possible that the observed diffusion characteristics were a result of competing effects, such that cell death and increased diffusion may have been more than compensated for by the reduction of extracellular space (therefore reduced diffusion). However, in recent MR spectroscopy studies, metabolic indicators for a significant response to therapy (decreased metabolite concentrations, particularly choline, and increased lipids due to cell membrane breakdown) were generally not observed.30 Therefore, the often-noted reduction of lesion volume after therapy is less likely to be caused by a loss of tumor cells. More likely is a tumor volume reduction due to reduced vasogenic edema consistent with the transient clinical improvement in DIBSG.
In this study, ADC decreased after RT in all patients with DIBSG. However, the extent of the decrease did not correlate with ΔTEFS after therapy. Indeed, there was a tendency toward longer survival in patients in whom a more substantial drop of ADC was observed, though significance was not reached.
A significant positive correlation between ΔFA and ΔTEFS was observed at the caudal midbrain level. In other words, patients with the most pronounced change from isotropic toward anisotropic diffusion had the longest ΔTEFS. All patients in our study received steroids at the time of diagnosis, with the intention of removing extracellular water and reducing peritumoral edema. Several studies have shown significant decreases in ADC in both peritumoral edema and the solid component of the tumors between 48 and 72 hours after steroid administration.31,32 In addition, a recent study has shown significant ADC drop in gliomas treated by Avastin (bevacizumab), whose antitumoral effect is still controversial.33,34 Therefore, our interpretation is that vasogenic edema has been met effectively with anticancer therapy and steroids, by improving the vascular structure and the leakiness of blood vessels.
ADC and FA of Individual Anatomic Regions
Our study indicates that diffusion characteristics at the caudal midbrain are most relevant in respect to prognoses. Baseline ADC and FA in the caudal midbrain showed the most significant correlation with overall survival. In addition, of all posttherapy changes, only ΔFA at the caudal midbrain level correlated with ΔTEFS. This observation suggests that a careful analysis of all substructures involved is of importance for optimal patient management. The reason for the significance of the caudal midbrain is unclear. It could be that microenvironmental factors (“seed and soil”) cause tumors involving caudal midbrain structures to be particularly infiltrative or to transition, with a higher likelihood, from lower grade to higher grade lesions. It has also been shown that tumors originating from different locations vary in their genetic profile,9 and the caudal midbrain may be associated with more malignant subtypes.
Is There an Added Value in Measuring FA?
A standard diffusion-weighted MR imaging scan is very fast and can be accomplished typically within 1 minute of total scanning time. DTI, particularly with a large number of diffusion directions, can take several minutes. This may stress young patients because of the high noise level during a longer period. However, in a previous study, it was demonstrated that FA was more sensitive than ADC in demonstrating tumor infiltration in supratentorial gliomas.35 In this study, we found that only ΔFA (in the caudal midbrain) correlated with ΔTEFS. While the number patients enrolled in this study was small and prospective confirmation is clearly needed, this finding indicates that there might be added value in performing more complex DTI studies instead of diffusion-weighted imaging alone.
Limitations
Apart from the small number of patients enrolled, a result of the low incidence of the disease, a number of other limitations of this study need to be acknowledged. First, histologic confirmation of the tumor type was available only in 1 subject, whereas no biopsies or postmortem examinations were available for the remaining subjects. Therefore a definitive correlation of diffusion changes with tumor biology was not possible at this point.
Second, a possible confounding factor is the differences in how subjects were treated. All patients underwent standard-dose radiation therapy, but there were differences in respect to chemotherapies (type and duration) that were administered to individual patients. One might speculate that initial imaging would better correlate with outcome if patients were all treated equally. A separate analysis of the 5 patients (subjects 1,2,4,5,6) who were treated identically (not reported in detail) did not, however, indicate that the correlation would improve if a more homogeneous study group were available. Also, the impact of steroids on diffusion properties of the lesions in patients who are treated at the same time is unknown. All patients received steroids (dexamethasone) at initial diagnoses. Doses were subsequent to therapy, adjusted and leveled off to the extent possible in individual patients. With DTI performed typically every 3 months, there is the possibility of underdetected changes in ADC and FA, particularly during initial RT.
Finally, for advanced imaging such as DTI to become useful for patient management in clinical settings, methods need to be used that are both robust and reproducible. However, approaches for obtaining mean ADC, ADCmin, ADCmax, mean FA, FAmin, and FAmax are variable and sensitive to user bias. For example, in 1 study the ADCmin was obtained from 5 to ten 40- to 60-mm2 regions of interest within solid components of the tumor on the ADC maps.14 Other groups obtained ADCmin by placing regions of interest in the solid tumor components on each contiguous section and selecting the lowest ADC value by visual inspection.26,36 In our study, we outlined the solid tumor component at 5 readily recognizable anatomic levels and obtained ADC and FA values for these regions. Obtaining ADC and FA from these relatively large areas will result in an overestimation (underestimation) of ADCmax and ADCmin and FAmax and FAmin. On the other hand, this method is fast and involves only a minimum of subjectivity (avoiding cystic, necrotic, and hemorrhagic areas) and is thus robust and reproducible.
Conclusions
ADC and FA values of diffuse intrinsic brain stem gliomas at presentation were consistent with tumors with extensive edema formation. There was a significant correlation between initial diffusion characteristics and survival. Patients with tumors with higher diffusivity and more isotropic diffusion tended to live longer. After therapy, diffusion was more restricted and became more anisotropic. This is not consistent with a significant response to therapy and may mainly reflect reduced edema. Of the posttherapeutic changes, only ΔFA in the caudal midbrain correlated with event-free survival. This suggests that anatomic considerations are important when reviewing DTI data and demonstrates the added value of performing DTI.
Acknowledgments
We thank Julia Castro and Arabhi Nagasunder for assistance with the review of medical records and manuscript preparation and Jane Tavare for help with the statistical analyses.
Footnotes
-
Indicates Fellows’ Journal Club selection
-

-
This work was supported by grants from Ian's Friends Foundation, the Thrasher Research Fund, the National Childhood Cancer Foundation/NIH/NCI (phase 1 and pilot consortium grant), and the Rudi Schulte Research Institute.
-
Paper previously presented at: 94th Scientific Assembly and Annual Meeting of the Radiological Society of North America, November 30-December 5, 2008; Chicago, Illinois.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received February 17, 2010.
- Accepted after revision April 15, 2010.
- Copyright © American Society of Neuroradiology