Abstract
BACKGROUND AND PURPOSE: While considerable attention has been directed to reducing the x-ray dose of individual imaging studies, there is little information available on the cumulative dose during imaging-intensive hospitalizations. We used a radiation-sensitive badge on 12 patients admitted with SAH to determine if this approach was feasible and to measure the extent of their x-ray exposure.
MATERIALS AND METHODS: After obtaining informed consent, we assigned a badge to each of 12 patients and used it for all brain imaging studies during their ICU stay. Cumulative dose was determined by quantifying exposure on the badge and correlating it with the number and type of examinations.
RESULTS: The average skin dose for the 3 patients who had only diagnostic DSA without endovascular intervention was 0.4 Gy (0.2–0.6 Gy). The average skin dose of the 8 patients who had both diagnostic DSA and interventions (eg, intra-arterial treatment of vasospasm and coiling of aneurysms) was 0.9 Gy (1.8–0.4 Gy). One patient had only CT examinations. There was no effort made to include or exclude the badge in the working view during interventions.
CONCLUSIONS: It is feasible to incorporate a film badge that uses a visual scale to monitor the x-ray dose into the care of hospitalized patients. Cumulative skin doses in excess of 1 Gy were not uncommon (3/12) in this group of patients with acute SAH. This approach could provide a measure of the cumulative dose and is a convenient tool to quantify the effect of dose-reduction strategies.
Abbreviations
- ACR
- American College of Radiology
- AP/LAT
- anteroposterior/lateral
- CTA
- CT angiography
- CTP
- CT perfusion
- DSA
- digital subtraction angiography
- DX
- diagnostic
- Fluoro.
- fluoroscopy
- ICU
- intensive care unit
- IR
- interventional radiology
- MCA
- middle cerebral artery
- PcomA
- posterior communicating artery
- RICA
- right internal carotid artery
- SAH
- subarachnoid hemorrhage
- SCA
- superior cerebellar artery
During their hospitalization, patients with SAH receive x-ray radiation from multiple sources. While there is the potential for both immediate and long-term effects from this x-ray exposure, there is currently no method in common use that measures a patient's cumulative dose in the hospital. We used a commercial film-based device (RADView; ISP, Wayne, New Jersey) to monitor the total x-ray dose to the head during the hospital stays of 12 patients after nontraumatic SAH. Our goals were to determine how this device could be incorporated into the clinical environment and to provide a measure of potential ranges of cumulative x-ray dose in this patient group, specific for the treatment algorithm of our hospital.
Materials and Methods
This study was approved by our institutional review board. A radiation-sensitive film badge (RADView) was used to quantify the absorbed x-ray dose to the head during the patient's hospitalization. The study was initiated after training our CT and angiography technologists to apply the badge for each x-ray study. Informed consent was provided for this investigation by the patients or their immediate families.
Badges and consent forms were available for 12 patients who were enrolled after admission to our institution with the diagnosis of SAH. Their average age was 49 years with a female/male ratio of 11:1. A unique badge was assigned to the patients at the beginning of their hospital stay. To collect data from these 12 patients, we enrolled 17 patients, but the badges of 3 patients were lost during their hospitalization and 2 other patients withdrew or refused consent. Badge loss occurred early in the study, and we tried to minimize losses by limiting the study to the duration of the patients' ICU stays. The last 5 consecutive badges were recovered. One patient (24/25) had 2 sequential badges issued during their hospitalization to ensure capture of early data.
Ten of the 12 patients had ≥1 cerebral aneurysm detected on imaging. There was 1 death (case 27) among these patients. One or more endovascular interventions were performed in 8 of the 12 patients.
The device uses a self-developing GAFCHROMIC film (ISP, Wayne, New Jersey), and the badge is transparent to x-ray. It is designed to indicate, with a visual scale, exposure to radiation within the range of 0.5–5 Gy in 0.5-Gy increments. This particular badge was found to be within 10% of dose measured against a standard film HPS N13.11–2001 by the University of Wisconsin Medical Radiation Research Center (personal communication from Heather Kisch, ISP, Wayne, New Jersey ; March 9, 2010).
For this study, rather than using the visual scale that reads in 0.5-Gy increments, we chose to use a more precise dose analysis provided by the manufacturer. This analysis was done completely blinded to patient information or record of x-ray exposures. The pixel value of the film, measured by using a flat bed scanner, was matched to a sensitometric curve specific to that badge film lot and generated by using known radiation doses. Each analysis indicated minimum, maximum, and average doses based on readings from each of the 6 regions on the badges.
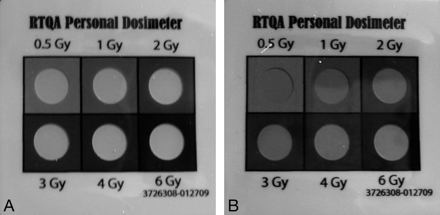
Because the badges are mildly light sensitive, each badge was covered in an opaque sheath to eliminate any contribution from light and the possibility that the readings themselves might influence clinical imaging decisions during the study. One badge was uncovered after recovery and photographed before analysis (Fig 1).
A, On this unused badge, all 6 circles are visible with good contrast with the background. B, This badge was used during 1 patient's hospitalization to measure dose. Note that the upper left circle matches the background along the left edge. The upper middle circle is visible. The visibility indicates that the measure of skin radiation dose at this point of hospitalization is more than 0.5 Gy but less than 1.0 Gy.
Our goal was to record exposure from all in-hospital x-ray examinations. However, because of the inherent delay in obtaining informed consent and examinations performed before transfer from other hospitals, some CT or CTA examinations were not captured during the patients' hospital stay. Also, because the badge was retrieved at the end of the patient's ICU stay, any additional imaging that was performed during the hospital stay was not captured.
The badge was placed directly on the patient's head before each neuroimaging examination, just cephalad to the right ear if possible. It was held in place during the examination by using a stabilizing strap or the elastic of a surgical hat and then removed and stored in a clearly marked receptacle near the patient. The badges are invisible on x-ray examinations, including CT, and no attempt was made to include or exclude the badge in the working view of interventional procedures.
Our imaging techniques were modified during the study. After receiving the dose calculations from the first 5 badges, we altered the scanning technique used for our portable CT scanner, to reduce the dose.
Results
With this approach, analysis of the badges revealed a range of cumulative x-ray dose of 0.22–1.8 Gy. The Table lists all captured x-ray procedures and the length of hospital stay for each patient. The average absorbed dose for the 3 patients who had no interventional procedures was 0.4 Gy. The average dose for those 6 who had either interventional treatments for vasospasm or aneurysm coiling was 0.9 Gy. The maximum dose we recorded was 1.8 Gy in a patient who had 10 CT scans, 1 diagnostic DSA examination, and 1 intervention during the ICU stay.
Patient skin dose and procedures
The measured exposure for each badge was offered as average, maximum, and minimum. The average value is listed in the Table, but the variation detected between the maximum and minimum doses on the badges from the 3 patients with doses above 1 Gy was ≤9%.
There was poor correlation between the total minutes of fluoroscopy and the measured dose. The patient with the highest dose, 1.8 Gy, was exposed to 41 minutes of fluoroscopy, while another with 61 minutes of total fluoroscopy time had a skin-absorbed dose of 0.6 Gy.
There was good correlation between dose and the total number of CT examinations and the length of stay in the ICU. The patient with a dose of 1.8 Gy also had the highest number of CT scans. The lowest dose measured, 0.22 Gy, occurred in a patient who had only CT scans during the short ICU stay. The 4 patients with the highest measured doses had the longest ICU hospitalizations (17–36 days).
Discussion
Diagnostic imaging and endovascular treatments that use x-ray have improved outcomes for patients with SAH. The significant role of these tools in current management and immediate concerns about patient mortality and morbidity to some degree lessen consideration of the effects of radiation dose during a hospitalization for SAH. While the survivors may have a decreased overall life expectancy,1 it is nevertheless important to consider both the immediate and long-term effects of radiation.
Recent publications have brought attention to the increased use of diagnostic CT. The number of CT scans in the United States alone has increased from 3 million per year in 1980 to 62 million in the past year.2 There is reasonable concern about the overall effects of this x-ray exposure, with predictions of 4000 additional cancers from CT scans of the head performed in 2007 alone.3 The risks of radiation can be divided into long-term carcinogenic effects and immediate effects such as hair loss.
The unit of measure used to quantify the absorbed x-ray dose is the gray, equivalent to 1 joule of energy per kilogram. Temporary hair loss is expected at an absorbed dose of 3 Gy, and permanent loss, at 7 Gy. This unit should be considered with the understanding that a single head CT scan is expected to produce a skin dose of approximately 0.05 Gy.
Many neurointerventional procedures will frequently exceed 1 Gy locally. Two investigations reported average procedural skin doses of 1–1.5 Gy.4,5 Reduction of radiation dose from each individual diagnostic and interventional exposure is critical for minimizing total patient and, thereby, population dose. While many publications suggest strategies to this end,4,6,7 few address the issue of cumulative dose during the hospitalization.
Patients with SAH are exposed to x-ray repeatedly for a short time, and the potential for cumulative effects exists. For example, Imanishi et al8 investigated the unexpected occurrence of bandlike hair loss in 3 among 44 patients, all examined with the same CTP technique. They found that all 3 patients with hair loss also underwent ≥2 cerebral angiographies. Their implication was that CTP alone could not account for the hair loss experienced by these patients. While the absorbed dose from CTP is high relative to a diagnostic CT, under the usual circumstances, it would never approach the 3-Gy threshold for temporary hair loss. For example, even by using a high-dose technique of 120 kV/200 mA for CTP, skin dose would not be expected to exceed 2 Gy.9 The report of Imanishi et al of hair loss in patients who had both DSA and CTP examinations provides some evidence for a cumulative dose effect when multiple x-ray imaging tests are used for a short time.
The cumulative skin dose we report for the patients in this study we believe is conservative. For patients transferred from other hospitals or because of consent delays, some initial CTAs were not included. None of our patients had CTP examinations that are known to contribute substantially to local skin dose.
There are other reasons to believe that the maximum skin dose will be higher. The angle of incidence of the x-ray beam on the badge may influence its measurement on the film. Because many of the DSA runs used oblique angles, their contribution to dose may be undervalued by this approach. In addition, the study of DSA by Schueler et al10 found that the maximum skin dose during diagnostic angiography occurs in the back of the head, not on the side where we positioned the badges in this study.
The ACR committee, in their report on radiation in medicine, recommended, “The ACR should encourage radiology practices to define a surveillance mechanism to identify patients with high cumulative radiation dose due to repeat imaging.”11 The estimated patient dose based on calculations or historic precedents has been used in some recent studies of radiation dose largely because there were no available data on a patient-specific dose.
That appears to be changing because most current CT scanners and angiography suites offer calculated dose estimates based on imaging parameters. For CT, this is the CT dose index, and for DSA, the dose-area product. With that information, it should be possible to calculate a running total of patient dose each day. Even when one incorporates known imaging factors, this is still a calculation and assumes that the x-ray tube performs as specified. In practice, it would require some process to record the data and keep a running total, which may prove possible with improved information systems.
Our DSA hardware did not provide a calculated dose at the time of imaging for this patient group, and for that reason, we made no comparison of the actual badge readings with a calculated dose. For the last patient who happened to have only CT studies, the badge read of 0.2 Gy closely matched the expected total dose of the 4 CT scans, however.
The rationale for a patient badge is that it provides an actual measured skin dose at least in 1 region rather than a calculated prediction. The advantages of the particular device we used are that it is relatively inexpensive, invisible, and unobtrusive and it provides an immediate visual indication of dose in 0.5-Gy increments. With the indicated total dose on the badge, this could be estimated daily during a hospital stay and in that way potentially influence imaging decisions.
However, while a patient-specific badge would seem easy to use in principle, because it requires participation of many staff in multiple locations, this project did require time and attention to train staff at the outset. Of course, other devices are available to measure skin dose. Thermoluminescent dosimeters and radiosensitive indicators attached to a hood have been used in other studies to measure the patient-specific x-ray dose.12 These did not seem as readily integrated into the clinical scenario however.
There are benefits to monitoring patient cumulative dose actively. It amplifies any small increase or decrease of CT technique because the effects accumulate during multiple scans in a hospital stay. It would be trivial to add a suitable radiopaque marker to the badge to allow confirmation that the badge was included or excluded from the working view during interventional cases or CTP studies. A tool of this nature could have averted the problems encountered with an unexpected elevated dose from CTP that was recently widely reported by the press.13 While CT scans are usually performed with a standard technique, there may be a role for an especially low-dose follow-up examination in patients with high badge readings who require repeat studies. This seems particularly important for portable CT units in the ICU.
Whether a running total of calculated dose or an actual measure of skin dose as we have demonstrated, a metric for cumulative dose during hospitalization is not widely used. The greatest benefit of a real-time dose measurement may accrue from the Hawthorne effect. This phenomenon of human psychology means that the simple act of measuring a behavior can alter it in a beneficial way. Awareness of cumulative dose would provide positive feedback on efforts to reduce dose. For example, we decided to decrease the dose used for our portable CT scanner once the readings from our first 5 patients were available. As imagers are challenged with finding the right balance of dose and detail with x-ray techniques, cumulative dose should be considered along with dose from individual studies.
Conclusions
The cumulative x-ray dose should be considered during hospitalizations for SAH because these admissions are imaging-intensive, and cumulative skin dose frequently exceeds 1 Gy. Because of the potential to both under- and overestimate dose by using predicted values, we measured x-ray dose to the head by using a commercially available film dosimeter. Awareness of cumulative dose during hospitalization could influence treatment decisions and facilitate dose-reduction strategies for interventional and diagnostic imaging procedures.
Acknowledgments
We thank Kelly Sexton for her assistance with this manuscript.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received January 28, 2010.
- Accepted after revision April 21, 2010.
- Copyright © American Society of Neuroradiology