Abstract
BACKGROUND AND PURPOSE: In this small series, local intrasinus catheter-directed heparin infusion with or without balloon thrombectomy was safe in the treatment of dural venous sinus thrombosis (DVST). Although systemic anticoagulation (SAC) is the treatment of choice, there is a lack of consensus regarding the best treatment should SAC fail or be contraindicated. We present our institutional experience with 16 patients in whom failure of, or contraindication to, SAC occurred and who subsequently underwent intrasinus catheter-directed heparin infusion with or without balloon thrombectomy.
MATERIALS AND METHODS: A retrospective review of 16 patients ranging in age from 14 days to 77 years who had intrasinus catheter-directed heparin infusion was undertaken with 9 male and 7 female patients identified. Of these 16 patients, 4 (25%) had a contraindication to SAC and SAC failed in 12 (75%). Technically successful intrasinus infusion catheter placement was achieved in all 16 patients (100%). Mean duration of infusion was 3.3 days (range, 1–6 days). Adjunctive balloon thrombectomy was performed in 9 (56.3%) of 16 patients. No procedure-related mortality occurred.
RESULTS: Partial and complete sinus recanalization occurred in 10 (62.5%) of 16 patients and 1 (6.3%) of 16 patients, respectively. There were 3 deaths (18.8%) attributed to disease progression. At most recent clinical follow-up (mean, 9.3 months), 11 (84.6%) of 13 surviving patients were independent, with a modified Rankin Scale (mRS) score of 1 or less.
CONCLUSIONS: Local intrasinus catheter-directed heparin infusion with or without adjunctive balloon thrombectomy seems to be a safe and effective treatment of DVST in patients in whom SAC failed or in whom there was a contraindication to SAC. In addition, the risk for symptomatic intracranial hemorrhage may be significantly lower than intrasinus infusion of thrombolytics.
Dural venous sinus thrombosis (DVST) can be challenging to diagnose because of a broad spectrum of nonspecific clinical signs and symptoms and often subtle imaging findings. The incidence in adults is reported to be 3 to 4 cases per million, with approximately 75% of those being women.1 DVST is reported to occur in 0.67 per 100,000 children per year, with 97% of these younger than 1 year.2
First-line treatment in symptomatic cases is supportive care with systemic anticoagulation (SAC) in patients without contraindication.1–4 There is a lack of consensus, however, regarding the best treatment should SAC fail or be contraindicated. Although some case reports and series have demonstrated efficacy of intrasinus catheter-directed infusion of alteplase and urokinase with no or minor complications,5–7 others have shown hemorrhagic complication rates in up to 30% of cases.4 Recent studies have also demonstrated efficacy of the AngioJet rheolytic catheter device (Possis Medical, Minneapolis, Minn).8–10
In an ex vivo porcine arteriovenous shunt, Nunes et al10 performed local, catheter-directed delivery of heparin in amounts sufficient to inhibit platelet-dependent thrombosis, which were several orders of magnitude lower than that used to achieve therapeutic SAC (no prolongation of bleeding parameters).11 The purpose of this investigation was to evaluate the use of intrasinus catheter-directed heparin infusion in the treatment of DVST.
Materials and Methods
After obtaining approval through our institutional review board, we undertook a retrospective review of prospectively collected clinical and imaging data of 16 patients who underwent intrasinus catheter-directed heparin infusion at our institution from 2001 to 2008 (Table 1). These 16 patients represent 6.8% of the 236 patients diagnosed with DVST at our institution from 2001 to 2008.
Summary data for 16 patients undergoing intrasinus catheter–directed heparin infusion
Patients ranged in age from 14 days to 77 years (mean age, 27 years). Nine male and 7 female patients were identified. Of these, 4 (25%) of 16 patients had a contraindication to SAC and SAC failed in 12 (75%) of 16 patients. Contraindications to SAC included trauma in 3 of 16 patients with evidence of intracranial hemorrhage or solid organ injury, and a large (4.6 cm × 5.2-cm) right parietal lobe hemorrhagic venous infarct in 1 patient. Of the 16 patients, 3 (18.8%) presented with headache and seizure and progressed clinically to an unresponsive state. Imaging revealed hemorrhagic venous infarcts in 2 patients (patients #2 and #12) and multilobar, bihemispheric nonhemorrhagic venous infarcts in 1 patient (patient #7) (Table 2). SAC was considered to have failed in patients when neurologic decline continued, or if the development of a new neurologic deficit occurred after 24 to 48 hours of effective SAC had been documented (activated partial thromboplastin time [aPTT], 60–80 s).
Imaging comorbidities and determination of candidacy for SAC
It is our standard practice to use intrasinus catheter-directed heparin infusion with or without balloon thrombectomy for the treatment of DVST in patients who have continued to decline neurologically despite SAC. In only 1 patient did we use intrasinus thrombolytics because of the severity of her clinical course in an effort to exhaust all possible treatment options (patient #7). This patient continued to decline and died as the result of extensive DVST. No hemorrhagic complications occurred as a result of intrasinus thrombolytic use in this patient. The decision to use balloon thrombectomy is based on the ability to successfully navigate the balloon across the occluded sinuses and is generally only performed when multiple (≥2) sinuses are occluded. In the case of trauma where endothelial injury is suspected, nonocclusive DVST, or single sinus occlusion, catheter-directed heparin infusion alone is our preferred treatment option.
Technically successful multi-side hole infusion catheter or infusion wire placement was achieved in all 16 patients (100%). Heparin doses ranged from 100 U/h up to half the total dose necessary to achieve a therapeutic aPTT (60–80 s). The mean infusion rate was 400 U/h. The mean intrasinus infusion duration was 3.3 days (range, 1–6 days).
Adjunctive endovascular balloon thrombectomy/clot angioplasty was performed in 9 (56.3%) of 16 patients. Clot angioplasty was performed to produce a channel for intrasinus catheter-directed heparin infusion. A Fogarty (Edwards Lifesciences, Irvine, Calif) balloon (4F or 5.5F) was used in 3 (33.3%) of 9 patients, a HyperForm (Micro Therapeutics, Irvine, Calif) balloon (7 mm) was used in 5 (55.6%) of 9, and a Viatrac (Abbott Laboratories, Abbott Park, Ill) balloon (5 or 6 mm) was used in 2 (22.2%) of 9 patients. Of the 7 patients not undergoing balloon thrombectomy, all had single sinus DVST and 2 had adjacent traumatic skull fractures. No procedure-related mortality occurred.
Our institutional SAC protocol included an intravenous (IV) heparin loading dose of 80 U/kg in the absence of intracranial hemorrhage demonstrated with head CT. When the decision was made to give SAC in the setting of intracranial hemorrhage, the loading dose was not administered. A continuous IV heparin infusion at 18 U/kg/h was then used to maintain an aPTT between 60 and 80 s. Under our protocol, aPTT levels were drawn every 6 hours until 2 consecutive therapeutic ranges were documented (aPTT, 60–80s), and aPTT levels were monitored every 24 hours. One patient receiving SAC went on to have heparin-induced thrombocytopenia (HIT) during catheter-directed heparin therapy and was switched to systemic and catheter-directed argatroban. One patient went on to have a small, asymptomatic subdural hematoma adjacent to the sigmoid sinus (SS) secondary to localized extravasation after difficult catheterization.
Results
Headache and seizure were the most common presenting symptoms accounting for 8 (66.7%) of 12 and 5 (41.7%) of 12 nontraumatic presentations, respectively. Seizure was present in 3 (100%) of 3 patients who subsequently died (Tables 1, 3).
Imaging and clinical follow up for 16 patients treated with endovascular catheter-directed intrasinus heparin infusion
The transverse sinus (TS) was most commonly affected in 12 (75%) of 16 patients, followed by the superior sagittal sinus (SSS) in 10 (62.5%) of 16 patients. The sigmoid sinus (SS) and internal jugular vein were involved in 8 (50%) of 16 patients. The straight sinus was involved in 3 (18.8%) of 16 and the internal cerebral veins in 2 (12.5%) of 16 patients. Multiple dural sinuses were involved in 9 (56.3%) of 16 patients. Cortical venous thrombosis was evident in all patients with presentations other than trauma (12/16). Two of 3 patients with straight sinus thrombosis and 2 of 2 patients with internal cerebral vein thrombosis were children younger than or equal to age 2 years (Table 1).
During initial hospitalization, partial sinus recanalization occurred in 10 (62.5%) of 16 patients and complete recanalization occurred in 1 (6.3%) of 16 patients. Posttreatment disposition was home in 7 (43.8%) of 16 patients and to inpatient rehabilitation in 6 (37.5%) of 16 patients. There were 3 deaths (18.8%) during initial hospitalization because of disease progression despite uncomplicated intrasinus catheter placement for heparin infusion. Follow-up imaging after discharge (mean, 9.3 months; range, 1–33 months) was available in 12 (92.3%) of 13 patients. Complete recanalization occurred in 10 (83.3%) of 12 patients, and partial recanalization occurred in the remaining 2 patients (16.7%). At most recent clinical follow-up (mean, 9.3 months; range, 1–33 months), 11 (84.6%) of 13 surviving patients were independent with a modified Rankin Scale (mRS) of 1 or less (Tables 1, 3). Severe developmental delay was present in 1 patient (2 years old), secondary to cerebral infarction resulting from extensive DVST, and long-term acute care was required for 1 patient (18 years old) because of severe closed head injury after trauma (Table 3). Of the 3 deaths in this series, all presented with seizure and had evidence of hemorrhagic venous infarction or multilobar, bihemispheric, nonhemorrhagic venous infarction.
Illustrative Cases
Patient 4.
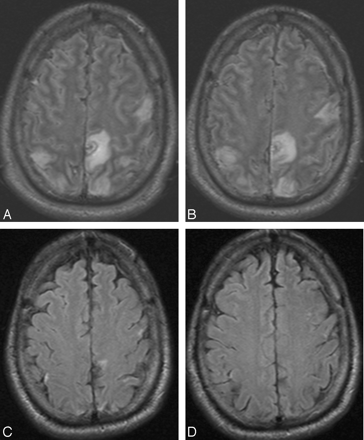
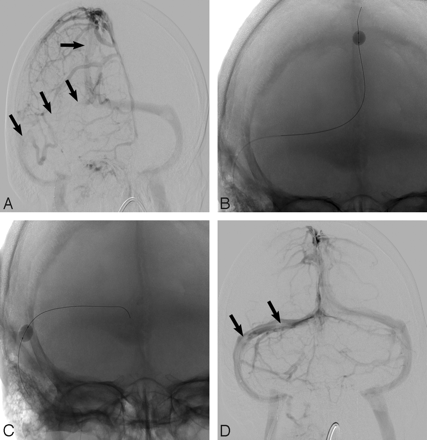
A 40-year-old white man presented to the emergency department with sudden onset of headache. The patient also had meningismus and a Glasgow Coma Scale score of 9. He underwent lumbar puncture and was diagnosed with bacterial meningitis. MR imaging and MR angiography revealed SSS thrombosis extending into the right transverse sinus and SS. Increased fluid-attenuated inversion recovery (FLAIR) signal intensity was also noted in the bilateral frontal and parietal lobes (Fig 1A, -B). The patient received systemic anticoagulation and, because of progressive neurologic decline, underwent conventional angiography (CA) 4 days later that revealed thrombosis within the SSS, TS, and proximal SS (Fig 2A). A 20 cm infusion length Cragg-McNamara infusion catheter (ev3 Neurovascular, Irvine, Calif) was placed from the SSS to the right jugular bulb, and intrasinus heparin infusion at 400 U/hr was initiated. Five days after balloon thrombectomy (Fig 2B, -C) and intrasinus heparin infusion, the patient was improving neurologically and significant recanalization of the TS was noted on follow-up CA (Fig 2B). The infusion catheter was removed, and the patient continued to recover neurologically. He was transferred to inpatient rehabilitation and was ultimately discharged home. A subsequent brain MR imaging examination performed 12 weeks later showed dramatic improvement in FLAIR signal intensity abnormality (Fig 1C, -D) and no evidence of DVST. He was able to return to an independent lifestyle and received warfarin (Coumadin; Bristol-Myers Squibb, New York, NY) on a maintenance basis for 6 months.
Four axial FLAIR MR images during hospitalization (A,B) and 12 weeks after (C,D) hospitalization for DVST. Multifocal FLAIR signal intensity abnormality in the frontal and parietal lobes (A,B) is attributed to nonhemorrhagic venous infarction because of SSS and cortical vein thrombosis. After treatment and maturation of cortical venous collaterals, only minimal signal intensity abnormality remains in this neurologically intact patient.
Oblique frontal angiogram (A) demonstrates segmental visualization of the SSS and no significant opacification of the right TS and proximal SS because of DVST (arrows). Balloon thrombectomy (B, C) of the SSS and right TS was performed, and after 5 days of intrasinus heparin infusion, a frontal angiogram (D) demonstrates near-complete flow restoration with only minimal residual filling defect (arrows; D) in the right TS.
Patient 1.
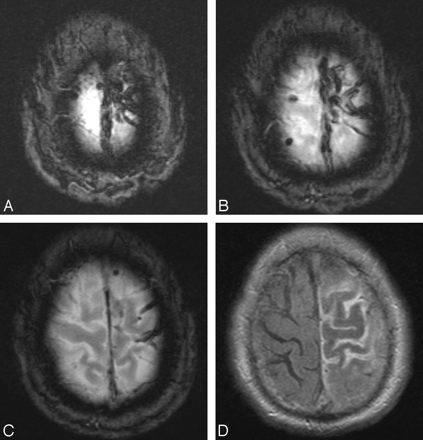
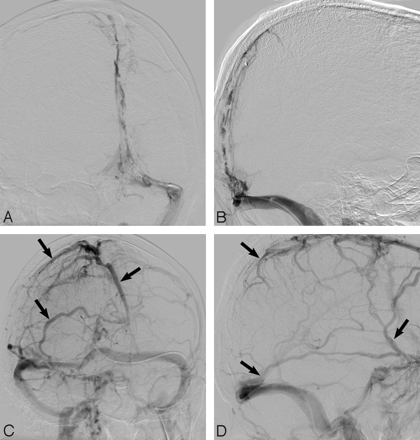
A 76-year-old white man presented to the emergency department with right-sided paresthesias and weakness. Brain MR imaging examination revealed increased FLAIR signal intensity in the left frontal and parietal lobes, including the motor and sensory strips, with diagnostic findings of SSS thrombosis on gradient-recalled echo imaging (Fig 3). Despite initiation of SAC, the patient, on hospital day 2, went on to have right hemiparesis and altered mental status. He underwent conventional angiography, which confirmed complete thrombosis of the SSS with cortical vein thrombosis. An intrasinus infusion catheter (Cragg-McNamara; ev3 Neurovascular), with an infusion length of 20 cm, was placed across the length of the sinus filling defect, and heparin infusion was commenced at 400 U/hr. On infusion day 3, the patient was diagnosed with HIT. Both systemic and local infusions were subsequently changed to argatroban, which was infused at a rate of 2 μg/kg/min. The patient improved clinically on days 4 through 7, and the infusion was discontinued on day 7. At that time, only minimal recanalization of the posterior one third of the SSS was evident on contrast injection through the infusion catheter (Fig 4A, -B), though cortical and diploic collateral venous channels were evident (Fig 4C, -D). After transfer to inpatient rehabilitation, the patient was discharged home neurologically intact, and argatroban was converted to warfarin (Coumadin; Bristol-Myers Squibb, New York, NY) for 6 months. At a 7-month follow-up visit, the patient had no neurologic deficits despite CT angiography, which demonstrated a persistent, nonocclusive filling defect in the SSS.
Three sequential axial gradient-recalled echo images (A-C) demonstrate characteristic “blooming” of SSS and cortical vein thrombosis (tarantula sign). Axial FLAIR image (D) shows swelling of the left frontal and parietal gyri with increased signal intensity in the adjacent sulci attributed to venous congestion.
Frontal oblique and lateral angiographic views (A,B) demonstrate extensive SSS, torcular, and TS filling defects in keeping with DVST. After balloon thrombectomy and 7 days of intrasinus heparin infusion, despite only minimal recanalization of the SSS, robust cortical venous flow (arrows; C,D) is evident in this neurologically intact patient. Note recanalization of the TS and SS.
Discussion
Recognition and prompt diagnosis are paramount for good clinical outcomes in patients with DVST. Confident diagnosis has been facilitated by many advances in noninvasive vascular imaging techniques such as 2D time-of-flight and 2D and 3D phase contrast MR angiography. Contrast-enhanced 3D MR venography has the advantage of superior vascular visualization with suppression of background signal intensity and no in-plane saturation effects.12 CT venography offers more widespread availability and rapid image acquisition in patients who are unable to undergo MR imaging because of various contraindications; however, because of complex data postprocessing and exposure to ionizing radiation and iodinated contrast, MR imaging is more commonly undertaken when possible.12
Rottger et al13 reported the time-dependent changes of venous infarcts associated with DVST on MR imaging and reported that 50% of such infarcts are reversible. This is concordant with our findings of reversible FLAIR signal intensity/edema in 4 (57.1%) of 7 patients (Table 2). Prognosis in patients with DVST varies by clinical presentation, and mortality rates have been reported to range from 6% to 15%.14 Complications, including the development of pulmonary embolism and dural arteriovenous fistulas, can be divided into acute and chronic categories corresponding to their time course of occurrence.3
SAC is the mainstay of treatment1–4 and should be initiated as soon as possible. Although anecdotal success with multiple therapies including catheter-directed thrombolytics (urokinase and alteplase),5–7 and rheolytic catheter and balloon thrombectomy8–10 have been reported, there remains a lack of consensus regarding the best treatment when SAC fails or is contraindicated. Furthermore, hemorrhagic complication rates have been reported in up to 30% of patients treated with catheter-directed thrombolytics.4 We only used intrasinus, catheter-directed urokinase in 1 of 16 patients (patient #7). This patient presented obtunded with multilobar, bihemispheric nonhemorrhagic venous infarcts and died before follow-up imaging was obtained.
Given the ability of locally infused heparin to raise antithrombin-III levels without elevation of systemic bleeding parameters,11 and general consensus that systemic heparinization is the mainstay of treatment, even in the presence of intracranial hemorrhage,4 local infusion of heparin should provide a safer alternative to locally infused thrombolytics. In the event of development of HIT, the use of argatroban seems to be efficacious, though this was only used in one of our patients. Anticoagulation provides additional benefits such as ease of management and monitoring, rapid reversibility, and familiarity among physicians, nurses, and other medical staff compared with thrombolytic agents. Furthermore, standardized monitoring protocols for systemic heparin infusion are common practice in all intensive care units, which increases the ability to titrate aPTT levels. If necessary, anticoagulation therapy may also be discontinued before other necessary procedures.
We prefer femoral vein access, which has a decreased risk for puncture site complications such as carotid arterial injury, compressive airway hematoma, and increased radiation exposure to the interventionalist, as can occur with jugular vein access. When dural sinus catheterization is not successfully achieved because of challenging anatomy or inability to pass the site of thrombosis, we elect to proceed with jugular vein puncture under sonographic guidance. In a study including 173 patients in a neurology intensive care unit, the incidence of primary bacteremia was 19%.15 As a precaution, we prefer to prescribe all patients with indwelling cerebral venous catheters prophylactic antimicrobial therapy.
Moreover, our approach is to remove the catheter when either clinical improvement is evident or angiographic improvement is seen in patients who cannot be adequately evaluated clinically because of severe neurologic injury. Once the catheter is removed, appropriate patients are converted to warfarin therapy for at least 4 to 6 months after discharge.
In the setting of DVST involving multiple dural sinuses, our preference is to provide a channel via balloon thrombectomy/clot angioplasty to facilitate distribution of catheter-directed heparin within the thrombosed sinuses. Balloon thrombectomy was performed before infusion catheter placement in 8 of 9 patients with DVST in 2 or more dural sinuses. In the other patient, a balloon could not be successfully navigated through the sinus occlusion (patient #16). Of these patients, 8 (88.9%) of 9 had presentations other than trauma. Of the 7 patients with DVST in a single sinus, only 1 patient (patient #10) underwent balloon thrombectomy. With this exception, we do not routinely augment catheter-directed heparin infusion with balloon thrombectomy for DVST isolated to a single sinus.
Of the patients treated with both catheter-directed heparin infusion and balloon thrombectomy (9/16 patients), there were 3 deaths because of disease progression, and 2 patients had permanent neurologic deficits (mRS, 4) because of traumatic brain injury (patient #10) and delayed diagnosis with cerebral infarction (patient #15). The remaining 4 patients live an independent lifestyle. Of the patients with DVST involving a single dural sinus who underwent catheter-directed heparin infusion, 7 of 16 went on to full recovery and live independently.
In our series, 4 patients presented secondary to traumatic injury. Of these, isolated thrombosis of the TS occurred in 1 of 4 patients and isolated thrombosis of the SS occurred in 2 of 4 patients while thrombosis of both the TS and SS was present in 1 of 4 patients. In the event that skull fractures were noted crossing the involved sinus, catheter-directed heparin infusion was performed without adjunctive clot angioplasty. The lesser burden of sinus occlusive disease seems to be much more amenable to treatment, and as such, outcome was excellent in this group (mRS, 0 in 75%) with only 1 patient experiencing lasting deficits, which were attributed to traumatic brain injury.
Of 16 patients treated with catheter-directed, intrasinus infusion of heparin, 11 (68.8%) returned to an independent lifestyle (mRS ≤ 1) at mean follow-up of 9.3 months (range, 1–33 months). Although final CA during initial hospitalization revealed complete and partial sinus recanalization in only 1 (6.3%) of 16 patients, and 10 (62.5%) of 16 patients, respectively, at last clinical follow-up, 11 (84.6%) of 13 surviving patients had a complete recovery and live an independent lifestyle (mRS ≤ 1). In addition, complete and partial recanalization was present in 10 (83.3%) of 12 patients and 2 (16.7%) of 12 patients, respectively, on follow-up imaging performed after discharge.
On analysis of our data—excluding the 3 patients who rapidly declined to an unresponsive state clinically and who had either very large hemorrhagic, or multilobar, bihemispheric nonhemorrhagic venous infarcts and subsequently died—our series shows complete recovery to an independent lifestyle for 11 (84.6%) of 13 patients. The remaining 2 patients live a dependent lifestyle attributed to extensive cerebral injury secondary to DVST (patient #15) and traumatic brain injury (patient #10) (Table 3).
Although this retrospective series is too small, and by design inadequate, to draw definitive conclusions regarding outcome related to individual causes of DVST, we found that pediatric patients, patients without SSS thrombosis, and patients with DVST related to traumatic injury had an overall better prognosis, whereas patients presenting with rapid clinical decline to an unresponsive state with diffuse DVST, including thrombosis of the SSS, progressed to mortality.
Conclusions
Local intrasinus catheter-directed heparin infusion with or without adjunctive balloon thrombectomy/clot angioplasty seems to be a safe and effective treatment of DVST in patients who are not candidates for SAC or in whom a trial of SAC has failed. With regard to patients with skull fractures crossing a thrombosed sinus, heparin infusion without clot angioplasty seems to be safe. In addition, intrasinus heparin infusion may present a significantly lower risk for symptomatic intracranial hemorrhage than intrasinus infusion of thrombolytics.
Footnotes
Previously presented as a scientific poster at: Annual Meeting of the American Society of Neuroradiology, May 16–21, 2009, Vancouver, British Columbia, Canada.
References
- Received February 13, 2009.
- Accepted after revision April 19, 2009.
- Copyright © American Society of Neuroradiology