Abstract
BACKGROUND AND PURPOSE: Although cement augmentation has been described in the literature for the treatment of benign sacral insufficiency fractures, only a few case reports have described the procedure's usage in the treatment of malignant lesions. The purpose of this study was to evaluate the feasibility, effectiveness, safety, and clinical outcome for percutaneous cement augmentation of patients with malignant lesions in the sacrum and pelvis.
MATERIALS AND METHODS: A prospective study of 12 patients (7 men and 5 women) with a median age of 64.5 years was conducted under appropriate institutional review board protocol. Patients had different types of malignant metastatic lesions of the sacrum and pelvic bones. All but 1 patient underwent preprocedure CT and MR imaging. All patients had a postprocedure CT, and all but 1 had sacral lesions. Six patients had a second lesion in the iliac bones. Under CT guidance, percutaneous cement augmentation was performed in 8 cases and under fluoroscopy guidance in 2 cases. In 2 cases, needles were placed under CT guidance, and the injection was performed under fluoroscopy. In 5 patients, a single needle was used; in another 5 patients, 2 needles were used. One patient had 3 needles, and another patient required 4 needles.
RESULTS: Adequate cement deposition was seen in all cases. Three patients had minimal clinically insignificant cement leakage. All treated patients (except 1 patient) reported decreased pain level with use of the visual analog scale (VAS) within 2 to 4 weeks of follow-up. No other subsequent surgical interventions were required.
CONCLUSIONS: Percutaneous cement augmentation of metastatic lesions of the sacrum and pelvic bones is a feasible and safe technique that can be performed under CT or fluoroscopic guidance. The technique results in decreased pain relief on short-term follow-up that can allow patients to tolerate future treatment.
Sacroplasty (percutaneous cement augmentation) for sacral insufficiency fractures has been described in the literature in multiple reports.1–5 The procedure can be performed with either fluoroscopic or CT guidance. Percutaneous vertebroplasty and kyphoplasty have been extensively reported in the literature for both benign and malignant vertebral compression fractures, with excellent outcomes. However, very few reports have addressed the possibility of treating sacral and other pelvic malignant metastatic lesions through percutaneous cement injection for alleviation of pain. The purpose of this study was to investigate the clinical feasibility and effectiveness, as well as short-term results, of percutaneous cement augmentation for the treatment of such lesions.
Materials and Methods
Before this prospective study was initiated, institutional review board approval was obtained. The accompanying on-line Table summarizes the study population and includes basic demographics, primary cancer type, lesion location, technique used, additional procedures, preprocedural and postprocedural visual analog scale (VAS) scores, and postprocedure CT summaries. There were 12 patients (7 men, 5 women) with a median age of 64.5 years who were included in the study. The patients had different types of malignant metastatic lesions of the sacrum and pelvic bones. All patients except 1 underwent preprocedure CT and MR imaging. All patients had a postprocedure CT scan. All patients had sacral lesions except for 1 patient, who had a single lesion in the iliac crest. Six patients had a second lesion in the iliac bones. Two patients had an associated soft tissue extension causing displacement of the rectum and extension into the sacral neural foramina and sacral thecal sac. One patient had a lesion in the ischium.
Procedure Technique
Patients were referred to this treatment by the treating oncologists for pain control. Cementation was performed concurrent with chemotherapy or radiation therapy. Preprocedure CTs were evaluated for the location of the lesions, appropriate needle access route, and number of needles necessary. Patients with extensive soft tissue components were excluded. After the appropriate informed consent was obtained, patients were placed in the prone position, either in the CT suite or in the angiography suite. Standard antiseptic techniques were used. All procedures were performed with the patients under conscious sedation. In the first 2 patients, needles were placed under CT guidance, and the cement injection was performed under fluoroscopy (C-arm in the CT suite). Eight cases were performed under CT guidance (Fig 1) and 2 cases, under fluoroscopy guidance. All needles used were standard 13G Jamshidi needles. In cases in which the lesion was determined to be so large that it could not be filled through a single injection, more than 1 needle was used. In 5 patients, a single needle was used; in another 5 patients, 2 needles were used. One patient required 3 needles, and another patient required 4 needles placed under CT guidance. A directional bone filler device (KyphX; Kyphon, Sunnyvale, Calif) with a side hole was used in 2 cases. After skin infiltration with local anesthetic, the needles were advanced under CT or fluoroscopic guidance. After confirmation of adequate position of the needle tip, a biopsy was performed in all cases before cement augmentation with use of a 17G core biopsy needle (PerCuCut; E-Z-EM, New Hyde Park, NY). Cementation was then performed with use of Zimmer dough-type cement (Zimmer, Warsaw, Ind) and Biotrace sterile barium sulfate (Bryan, Wobum, Mass) or Confidence cement (DePuy Spine, Raynham, Mass). An average of 2 to 6 mL of cement was injected. Injection of cement was performed under direct fluoroscopic guidance when fluoroscopy was used. When CT was used, injection of cement was performed in small increments, and the patients were scanned in between injections. The injection was terminated when the operator felt that most of the lytic lesion had been filled with cement or when potential leakage or pressure over a vital structure was imminent. In 2 patients, there was tumor extension into a neural foramen and complaints of radicular pain, which were treated with selective nerve root block.
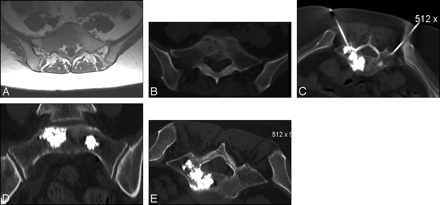
A 44-year-old woman with metastatic breast cancer to the sacrum and lumbar spine. A, Axial T1-weighted image showing the tumor extension with low signal intensity involving the right sacral ala and most of the body of S1 vertebra. B, Corresponding axial CT image showing the extent of the tumor. C, Axial CT image showing placement of 2 needles. D and E, Postprocedure CT images showing the final distribution of the cement. Patient VAS score went from 9/10 before the procedure to 2/10 at the follow-up visit.
Assessment of Pain Relief
Pain level was assessed with VAS scores taken both before the procedure and 2 to 4 weeks afterward during a follow-up visit. Patients were asked to rate their level of pain by using a 10-point scale, where 0 represented no pain and 10 represented the worst pain they had ever experienced.
Results
Adequate cement deposition was seen in all cases. Three patients had minimal clinically insignificant leakage (on-line Table). All treated patients reported decreased pain level by VAS (except for 1 patient) within 2 to 4 weeks of follow-up. Average VAS score was 8.6 before the procedure and improved to 3.8 after the procedure. No other subsequent surgical interventions were required, to my knowledge, to maintain improvement in any of the cases.
Discussion
Metastatic tumors are the most common malignant lesions to occur in the sacrum.6 However, tumors of the sacrum are quite rare overall, accounting for only 1% to 7% of all spinal tumors that come to clinical attention.7 Delay in diagnosis is common and may result from the unique properties of these tumors and their location, in particular, the capability of the sacral canal to permit asymptomatic expansion of the tumor. Surgical resection frequently presents an unusual challenge because of procedural morbidity.
Treatment of these tumors is typically palliative and is often achieved with radiation and chemotherapy alone. Surgery may be recommended if the patient has a life expectancy greater than 6 months and presents with a progressive neurologic deficit. The main goals of treatment are pain control and the restoration and maintenance of neurologic function.8,9 Selection of which surgical approach to take is dictated by the location of the lesion within the sacrum. Gross total resection is believed to be the best management approach as long as an acceptable functional result is anticipated.10 Sacral reconstruction is often required after resection of tumors invading S1 and S2 and the sacroiliac joints. Reconstruction is needed to provide early mobilization and to prevent instability.9
This study demonstrates that percutaneous cement augmentation of malignant lesions of the sacrum and the pelvis is a viable option among the various treatment options. Cementation allows local pain control and perhaps creates some stabilization. In this study, the procedure resulted in decreased VAS scores and early immobilization. This finding seems to be consistent with information known from the cementation of malignant lesions of the spine by vertebroplasty or kyphoplasty. Cementation does not affect the patient's other treatment regimens such as radiation therapy or chemotherapy. Further experience is still needed to evaluate whether cementation can alter surgical management or the current approach to these lesions by providing some stability.
Hierholzer et al11 proposed that internal reinforcement of the trabecular bones prevents the ongoing deformation of the bone itself, with consequent painful stress on the periosteum. Because bone pain within other areas of the skeleton is believed to follow the same pathologic mechanism (ie, activation of pain nerves of the periosteum), the authors hypothesize that stabilization of the fragile bone should lead to a similar analgesic effect. It is also postulated that the neurotoxic effect of monomer polymethylmethacrylate and the exothermic reaction produced during cement polymerization could cause periosteal denervation.12
One limitation of this study was the inability to obtain long-term follow-up. This has proved difficult for such a small sample, especially because most of those patients were presented late in the course of the disease. However, it was this same complication in this patient set that demonstrated the need for minimally invasive procedures that provide quick pain control and avoid surgical intervention.
Several authors have reported single cases with documented pain relief, in which the procedure was performed under either fluoroscopy or CT guidance.13–15 However, there is still a debate whether CT or fluoroscopic guidance is the superior technique for this procedure. Most of the procedures of this series were performed with CT guidance because of the complex nature of the lesions. Many lesions required more than 1 needle for adequate cementation. It is also essential to avoid important structures such as nerve roots. In the absence of CT fluoroscopy, the cement needs to allow for a long working time (preferably high-viscosity cement) to allow frequent imaging during injection. Recently, the long-axis technique has been described for fluoroscopy-guided sacroplasty in sacral insufficiency fractures.16 I believe that this technique can be used for focal lesions in the sacral alae if the tumors are contained inside the bone with no soft tissue extension and there is no involvement of important structures such as the neural foramina. Kelekis et al17 has described percutaneous fluoroscopy-guided techniques for an osteoplasty of the superior and inferior pubic rami and ischial tuberosity in 14 patients. Sacroplasty for sacral insufficiency fractures has been described by placing the needles under CT guidance. The patients were then transferred to the angiography suite for cement injection under direct fluoroscopy guidance.17 CT-guided fluoroscopy has also been described in vertebroplasty from metastatic diseases.18
This study has demonstrated that percutaneous cementation of sacral and pelvic metastases under CT or fluoroscopy guidance is a safe, effective, and feasible procedure that provides short-term pain relief. It can be performed as an adjuvant to radiation therapy and chemotherapy.
Footnotes
indicates article with supplemental on-line table.
References
- Received December 14, 2008.
- Accepted after revision February 2, 2009.
- American Society of Neuroradiology