Abstract
BACKGROUND AND PURPOSE: Previous studies have demonstrated limited benefit with endovascular procedures such as stent placement in octogenarians. We evaluated the safety and effectiveness of intra-arterial recanalization techniques to treat ischemic stroke in patients 80 years or older presenting within 6 hours of symptom onset.
MATERIALS AND METHODS: We pooled the data from 4 prospective studies by evaluating intra-arterial recanalization techniques for treatment of ischemic stroke. Clinical and radiologic evaluations were performed before treatment and at 24 hours, 7 to 10 days, and 1 to 3 months after treatment. We performed multivariate analyses to evaluate the effect of ages 80 years and older on angiographic recanalization, favorable outcome (modified Rankin scale of 0–2), and mortality rate at 1 to 3 months.
RESULTS: A total of 101 patients were treated in the 4 protocols. Of these, 24 were 80 years or older. There was no significant difference between the 2 age groups in sex, initial stroke severity, time to treatment, site of vascular occlusion, and rate of symptomatic and asymptomatic intracranial hemorrhage (ICH). In logistic regression analysis, age 80 years or older was associated with a lower likelihood of a favorable outcome (odds ratio [OR], 0.40; 95% confidence interval [CI], 0.13–1.2; P = .11) and recanalization (OR, 0.36; 95% CI, 0.12–1.1; P = .07) and with higher mortality rate (OR, 3.17; 95% CI, 1.05–9.55; P = .04) after adjusting for study protocol. After adjusting for recanalization in addition to study protocol, the older age group still had a lower likelihood of favorable outcomes (OR, 0.34; 95% CI, 0.1–1.1; P = .07) and higher mortality rates (OR, 3.62; 95% CI, 1.15–11.36; P = .027).
CONCLUSIONS: Our study demonstrates that patients 80 years and older are at higher risk for poor outcome at 1 to 3 months following intra-arterial recanalization techniques. This relationship is independent of recanalization rate and symptomatic ICH supporting the role of other mechanisms.
Octogenarians are one of the fastest growing population segments in the United States. Approximately 6% of the population are 80 years or older,1 and a threefold increase is expected by the year 2050.2 The average life expectancy for an 80-year-old is approximately 8 years.3 Patients who are 80 years or older have been shown to be at higher risk for complications after endovascular procedures such as carotid stent placement.4,5 Therefore, many investigators caution against the use of such endovascular procedures in elderly patients.5 Studies that have focused on the use of intravenous thrombolysis in patients 80 years or older have suggested an unclear risk-to-benefit ratio because of high rates of symptomatic intracranial hemorrhage (ICH) and poor clinical outcomes.6 However, other studies have refuted those findings.7 A report of 62 patients with acute ischemic stroke treated with a combined intravenous and intra-arterial approach found that mortality rate was higher in persons older than 80 years (63%) than among those 80 years or younger (11%)8; however, patients older than 80 years had a higher severity of initial deficits. The rate of symptomatic ICH was 25% and 6% in patients older than 80 years and in patients 80 years or younger, respectively. Because of high mortality and ICH rates in this group, patients older than 80 years were excluded from the Interventional Management of Stroke trials.9,10
There are limited data on the outcome of intra-arterial recanalization techniques in patients 80 years or older with ischemic stroke.11 Because of the observed and anticipated shifts in demographics and the increasing availability of endovascular interventions for acute ischemic stroke, it is important to understand the outcomes associated with such treatments in this patient population. We performed a retrospective analysis of prospectively collected data to provide further information on this issue.
Materials and Methods
For our analysis, we pooled data from 4 prospective protocols of endovascular treatment for acute ischemic stroke. The results of the protocols have been previously published.12–15 Before the treatment and at 24 hours after symptom onset, each patient was assessed by means of the National Institutes of Health Stroke Scale (NIHSS) by a neurologist. Routine laboratory testing included hemoglobin level, hematocrit, platelet count, electrolytes, and coagulation profile. A cranial CT scan was performed at presentation and immediately and at 24 hours after completion of endovascular therapy. Diagnostic and interventional angiographic procedures were conducted via the femoral approach. The individual protocols are described below.
Early neurologic improvement was defined as a reduction of 4 or more points in the NIHSS score at 24 hours compared with baseline. Outcome was determined for the surviving patients at 1 to 3 months postprocedure, either at the time of a clinic visit or in the setting of a telephone interview. Outcomes were evaluated with the modified Rankin scale [mRS]. Favorable outcome was defined as an mRS score of 2 or less.
Protocol 1: Intravenous Recombinant Tissue Plasminogen Activator and Mechanical Clot Disruption
Intravenous recombinant tissue plasminogen activator (IV rtPA) was administered at a dose of 0.9 mg/kg with the standard dosing protocol. Emergent cerebral angiogram was performed in patients with an initial NIHSS score of 10 or greater.12 After an arterial occlusion was documented, a 2.3F microcatheter was advanced to the occluded vessel and placed in the proximity of the thrombus over a microwire through the guide catheter. Mechanical disruption of the thrombus was undertaken in the large vessels, including the internal carotid artery (ICA), proximal middle cerebral artery (MCA, M1 segment), and basilar artery (BA), by primary angioplasty. The angioplasty balloon was undersized in reference to the diameter of the adjacent portion of the occluded vessel. One or, infrequently, 2 dilations of the angioplasty balloon were performed. In the smaller vessels, including the distal MCA (M2 and M3 segments), anterior cerebral artery (ACA), and posterior cerebral artery (PCA), a 4-mm snare (Amplatz Goose-Neck Microsnare; Microvena, White Bear Lake, Minn) was introduced through the 2.3F microcatheter, and multiple passes were made through the clot with use of the fully extended loop of the snare to induce fragmentation. Snare maneuvers were permissible for proximal vessels if the distal end of the thrombus extended into the distal vessels not amenable to angioplasty such as the M2 segment of the MCA. No intravenous heparin was used during the procedures.
Protocol 2: Intravenous Abciximab and Intra-arterial Reteplase
This protocol was applied to all patients who presented between 3 and 6 hours of symptom onset with an NIHSS score between 4 and 23.13 Once an occlusion was demonstrated by cerebral angiogram, a 0.25-mg/kg bolus of abciximab was administered intravenously followed by a 0.125-mcg/kg/min infusion (maximal dose, 10 mcg/min) for 12 hours. Intravenous heparin (15 U/kg bolus) was used to achieve an activated coagulation time (ACT) greater than 180 seconds. A 2.3F microcatheter (Prowler Plus; Cordis, Miami Lakes, Fla) was navigated into the occluded vessel, and 0.25 U of reteplase were injected over 5 minutes proximal to the thrombus. The microcatheter was then advanced into the thrombus, and another 0.25 U of reteplase (1 U/20 mL of saline) was administered over a 5-minute period (1 mL/min). Additional thrombolytic was injected as needed into the matrix of the clot in successive 0.25-U boluses. Serial angiograms were performed after each 0.25-U bolus of reteplase to evaluate for recanalization. The procedure was continued until either complete recanalization was achieved or the maximal dosage specified by the protocol had been reached.
Protocol 3: EKOS MicroLys North American Trial
Patients enrolled in the North American safety and feasibility study to evaluate the MicroLysUS infusion catheter (EKOS, Bothell, Wash)14 for which angiographic data were available for review were included in our analysis. Patients had an NIHSS score of 8 or more and were within 6 hours (suspected anterior circulation ischemia) or 24 hours (suspected posterior circulation ischemia) from symptom onset. The full set of inclusion and exclusion criteria for this trial has been described previously.4 After angiography had revealed an arterial occlusion, 2000 U of intravenous heparin was administered, followed by a 500-U/h infusion for 4 hours. Subsequently, the 2.5-F MicroLysUS 2.1 MHz sonographic-tipped infusion catheter (EKOS) was advanced through the guide catheter to the target vessel, and sonographic transmission at a power of 0.21 to 0.45 W was initiated. The same catheter was used to administer rtPA (2 mg bolus, followed by 0.3-mg/min infusion for 60–120 minutes to a maximal dose of 20–30 mg) or reteplase (0.4-U bolus, followed by infusion of 8 U for 60 minutes). Angiograms were performed at 15-minute intervals.
Protocol 4: Intra-arterial Reteplase and Mechanical Clot Disruption
This protocol15 included 46 consecutive patients, not eligible for intravenous rtPA who received intra-arterial reteplase supplemented by mechanical thrombolysis. The patients were deemed ineligible for intravenous rtPA if they had an NIHSS score of 16 or more, if they presented at more than 3 hours after onset, or if they had had major surgery in the 2 weeks preceding the stroke.
Intravenous heparin was administered as a 30-U/kg bolus. After an arterial occlusion was documented, a 2.3F microcatheter was advanced to the occluded vessel in the proximity of the thrombus through the guide catheter over a microwire. Intra-arterial reteplase (0.1-U/mL solution) was administered in boluses of 1 U up to a maximal dose of 4 U into the matrix of the clot. Mechanical disruption of the thrombus was undertaken in large vessels, including the ICA, proximal MCA (M1 segment), and BA by primary angioplasty. In the smaller vessels, including the distal MCA (M2 and M3 segments), ACA, and PCA, a 4-mm snare (Amplatz Goose-Neck Microsnare; Microvena) was introduced through the 2.3F microcatheter, and multiple passes were made through the thrombus with use of the fully extended loop of the snare to achieve clot fragmentation. Angiography was performed after each unit of reteplase or mechanical maneuver.
Evaluation of Imaging Data
Immediate pretreatment angiographic images were obtained and graded by a previously validated classification system on the basis of the location of the occlusion and collateral supply to the affected regions.16 The preprocedure and postprocedure angiographic occlusion was also graded with use of the Thrombolysis in Myocardial Infarction (TIMI) scheme.17 Recanalization was defined by either complete (TIMI 3) or partial (TIMI 2) flow in the distal arterial segment. CT scans performed at 24 hours after each procedure were reviewed for the presence of ICH. ICH was considered symptomatic if it was temporally related to neurologic deterioration, defined as an increase of 4 points or more in the NIHSS score compared with pretreatment evaluation. Asymptomatic ICH was defined with the criteria used by individual studies.
Statistical Analysis
Demographic, angiographic, and clinical variables were compared between patients 80 years and older and in those younger than 80 years. Continuous and categoric variables were presented as mean values along with SD and frequency, respectively. We confirmed that continuous variables were normally distributed. We compared means and frequencies using analysis of variance and χ2 methods, respectively.
The association between age older than 80 years and outcome variables (recanalization, early neurologic improvement, favorable outcome at 1–3 months, and mortality rates) was assessed by means of bivariate (χ2) and multivariate (logistic regression) analysis adjusting for study protocol.
We subsequently entered recanalization in the multivariate model evaluating the effect of age 80 years or older on favorable outcome and mortality rates at 1 to 3 months to determine its effect on the observed association. We performed all analysis using JMP statistical software (SAS, Cary, NC).
Results
A total of 101 patients were treated in the 4 protocols. The mean age (± SD) was 66.9 ± 15.9 years; 55 were men. A total of 24 patients belonged to the older age group, whereas 77 patients were younger than 80 years. The Table shows the demographic, clinical, and angiographic characteristics of the 2 groups.
Demographic and clinical characteristics according to age strata for patients with acute ischemic stroke treated with endovascular interventions
There was no significant difference between the 2 age groups as regards sex, severity of initial neurologic deficits (NIHSS score), site of arterial occlusion, interval between symptom onset and treatment, rate of early neurologic improvement, and asymptomatic and symptomatic ICH.
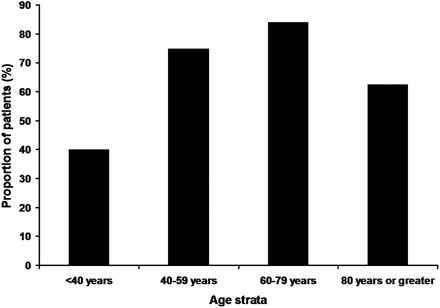
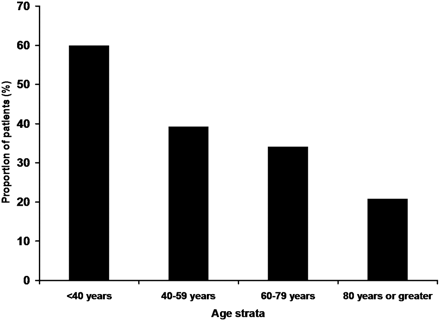
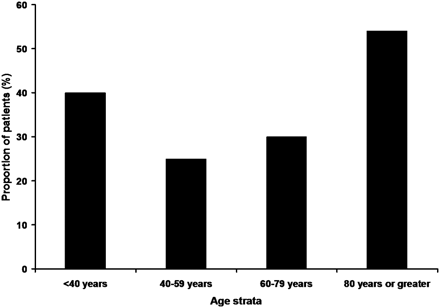
The younger patients were more likely to have partial or complete recanalization (60/77 vs 15/24; P = .13) and favorable outcome (29/77 vs 5/24; P = .13). However, both trends were not significant. Mortality rates were significantly higher in the older age group (54% vs 29%; P = .02). Figures 1–⇓3 show the rates of recanalization, favorable outcome, and mortality rate as a function of age strata.
The rates of partial or complete recanalization according to multiple age strata are demonstrated.
The rates of favorable outcomes at 1 to 3 months according to multiple age strata are demonstrated.
The rates of mortality at 1 to 3 months according to multiple age strata are demonstrated.
In multivariate analysis (controlling for study protocol) age 80 years or older was associated with a lower likelihood of angiographic recanalization (OR, 0.36; 95% CI, 0.12–1.1; P = .07) and favorable outcome (OR, 0.40; 95% CI, 0.13–1.2; P = .11) and with higher mortality rate (OR, 3.17; 95% CI, 1.05–9.55; P = .04). After adjustment for recanalization in addition to study protocol, age 80 years or older continued to be associated with a lower likelihood of a favorable outcome (OR, 0.34; 95% CI, 0.1–1.1; P = .07) and higher mortality rate (OR 3.62; 95% CI, 1.15–11.36; P = .027).
Discussion
Salient Findings of the Study
We found that intra-arterial recanalization techniques in patients aged 80 years or older 1) are less likely to result in recanalization; 2) are less likely to lead to favorable outcome at 1 to 3 months independent of recanalization; 3) are not more likely to be complicated by ICH than in younger patients; and 4) are associated with higher mortality rates regardless of recanalization. Our sample size was too small to achieve significance for our first 2 observations (P = .07 in both cases). Our findings suggest that endovascular treatment among patients 80 years or older is less beneficial in this population but not because of lower technical success rates or higher complication rates. In our multivariate analysis, we did not control for NIHSS score, time to treatment, sex, or location of arterial occlusion because they were evenly distributed among our 2 age groups (Table).
Our results are largely in agreement with those reported by Kim et al,11 who compared the baseline characteristics, complications, and outcomes of intra-arterial recanalization techniques in patients 80 years or older (n = 33) and contemporaneous patients younger than 80 years (n = 81). Lower rates of favorable outcome after thrombolysis in the elderly population are also reported in the cardiology literature. A meta-analysis of 11 published randomized clinical trials of fibrinolysis in acute myocardial infarction18 (sample size ≥ 3000 without age limit in recruitment) showed that patients 75 years or older had increased rates of mortality, ICH, and cerebrovascular events within 30 to 35 days after treatment (OR, 4.4). A pooled analysis19 of 3032 patients from the Primary Angioplasty in Myocardial Infarction (PAMI)-2, Stent-PAMI, and PAMI-No Surgery found a lower procedural success rate and a higher rate of in-hospital mortality in patients 75 years or older. Studies have also suggested that the poor outcomes in elderly patients after myocardial infarction are independent of myocardial reperfusion.20
Local Vascular And Thrombus-Related Factors
Several factors could play a role in the diminished recanalization rates among the elderly population. It is possible that elderly patients have a higher likelihood of atherosclerotic narrowing with a superimposed thrombus compared with younger patients, who are more likely to have embolic occlusion of a less diseased vessel. It has been shown that patients with more cardiovascular risk factors (and therefore presumably more atherosclerosis) have lower rates of recanalization.21 Occlusions of vessels more likely to have atherosclerosis such as the cervical ICA have lower rates of recanalization.22 In a similar fashion, the coronary arteries of elderly patients with myocardial infarction tend not to recanalize when treated with primary angioplasty.19,23 High atherosclerotic plaque burden has been associated with the microvascular no-reflow phenomenon24 and reduced tissue perfusion. However, it should be noted that a consistent association between the cause of the thrombus and recanalization rates has not been observed, particularly in the intracranial circulation. The influence of presumed cause of the thrombus on recanalization after treatment with intra-arterial thrombolysis was studied in 62 patients with intracranial ICA or MCA occlusion.25 The type of thromboembolus was atherosclerotic in 6 patients, cardioembolic in 29, of other determined cause in 4, and of undetermined cause in 23 patients. Recanalization was observed in 67% and 59% of the patients with thromboembolic occlusions because of large-artery atherosclerosis and cardioembolic sources, respectively. The tortuosity of the cervical vasculature increases with age, making all endovascular procedures more demanding technically and rendering the use of some devices practically impossible.26
Spontaneous thrombolytic activity is reduced in older patients.27,28 This is partly attributed to higher expression of one of the principal inhibitors of fibrinolysis, plasminogen activator inhibitor-one (PAI-1). Higher PAI-1 expression is found in a variety of conditions associated with the process of aging,29 including obesity. Adipose tissue is an important source of inflammatory cytokines and PAI-1.30 Dietary restriction leads to diminution of elevated PAI-1 levels and reduces inhibition of the fibrinolytic system in elderly, obese subjects.31 Lower levels of physical activity also lead to enhanced expression of PAI-1.32 In old aged persons, levels of circulating testosterone are low. Testosterone may affect thrombotic activity through its effect on nitric oxide production.33
Old age has been associated with poor recovery in patients with acute ischemic stroke who receive intravenous thrombolysis.34 Collateral circulation is believed to salvage parts of the ischemic territory and, thus, positively affect eventual functional outcome.16,35 In the elderly population, the ability to form collateral pathways may be acutely diminished. It has been shown that advanced age predicts the absence of collateral circulation to the ischemic region in acute myocardial infarction.36 In the same analysis, the absence of collaterals was an independent predictor of in-hospital mortality rates in patients 70 years or older but not in patients younger than 70 years. We did not detect an age-dependent difference in the severity of initial deficits or rates of early neurologic improvement. Poor outcomes may also be related to age-related reduced neural plasticity,37,38 limiting the capacity for neurologic recovery.
Medical Comorbidities
Previous studies evaluating patients admitted for cardiovascular disease–related procedures have found a higher rate of medical comorbidities and poor outcomes in patients 80 years or older.39–41 There is some evidence to suggest that medical comorbidities contribute to the poor outcomes observed in these patients. A study39 compared the clinical characteristics and in-hospital outcomes of 7472 patients 80 years or older with those of 102,236 younger patients who underwent percutaneous coronary interventions (PCI) at 22 hospitals. Patients 80 years and older had more comorbidities; more extensive coronary disease; and a twofold to fourfold increased risk for complications, including death, Q wave myocardial infarction, stroke, renal failure, and vascular complications. For elective procedures, patients in this age group had a nearly 10-fold higher mortality rate and were strongly influenced by comorbidities (0.8% mortality rate with no risk factors vs 7% with renal insufficiency or left ventricular ejection fraction <35%).
Another study40 population consisting of 4360 consecutive patients undergoing PCI in 7 major Australian hospitals found that patients 80 years and older (vs patients <80 years) had more comorbidities such as cerebrovascular disease, renal impairment, congestive heart failure, and chronic airway disease. These patients also had significantly increased 30-day mortality rates and rates of major adverse cardiac events. An analysis41 of the New York State Angioplasty Registry in emergency and elective PCI cohorts across 3 age categories (<60, 61–80, and >80 years) evaluated 10,964 patients who underwent emergency PCI and 71,176 patients who underwent elective PCI. Elderly patients had more comorbidities, including more extensive coronary atherosclerosis, hypertension, peripheral vascular disease, and renal insufficiency. In the emergency and elective PCI groups, in-hospital mortality rates and adverse event rates increased incrementally by age group.
Two studies42,43 have evaluated the rates of medical comorbidities and effect on clinical outcome in patients with ischemic stroke. Medical comorbidities were assessed with use of the Charlson Comorbidity Index.44 The index encompasses 19 medical conditions weighted 1 to 6, with total scores ranging from 0 to 37. A weight is then assigned to each condition on the basis of the relative risk and from the weighted conditions, and a sum score can be calculated to yield the total comorbidity score. One study42 estimated changes in rates of ischemic stroke and ICH, comorbidity profile, and case fatality rates in Quebec during 15 years. In this study (involving 101,831 persons with ischemic stroke and 11,215 persons with ICH), though age and comorbidity of the population increased, case fatality rates decreased with time. Age and type of stroke were strong predictors for early (7 days) and later (8–30 days) case fatality, whereas comorbidity was important only for later death. Another multicenter cohort study43 of 26,676 hospital admissions for ischemic stroke identified from 606 hospitals in Canada found that case fatality rates at discharge ranged from 6% for patients younger than 59 years to 24% for patients 80 years or older. Patients 80 years and older were less likely to be admitted to the intensive care unit and discharged to where they resided before the stroke occurred. However, difference in clinical outcome could not be attributed to a differential rate of medical comorbidities between the elderly and younger patients. The current data suggest that the impact of medical comorbidities on clinical outcome in patients with stroke is less prominent than that observed in patients undergoing PCI procedures. It is possible that the effect of comorbidities is highlighted in patients with ischemic stroke who undergo thrombolysis (vs those who do not) because these patients seem to have higher severity of disease.45
Study Limitations
The study was a pooled analysis of 4 different protocols. Therefore, there was heterogeneity in the data that we collected. We attempted to adjust for the different protocols in the multivariate analyses. The patients received different combinations of pharmacologic and mechanical thrombolysis. There is a possibility that elderly patients were treated less aggressively; however, all of the studies were prospectively designed, and there was uniformity in the procedures performed and data collected independent of patients’ age. However, data regarding underlying causes and associated systemic comorbidities were not collected; therefore, we were unable to analyze the differences in these variables between age groups and their effect on recanalization rates and outcome. Although all available angiographic images were reviewed, some important information such as proximal vascular tortuosity, extent of collateral formation, and transit times (indicative of microvascular patency), could not be assessed because of lack of standardized image acquisition. There were differences in the definitions of asymptomatic ICH within studies, which may have introduced some heterogeneity in the pooled analysis. Certain data such as serum concentration of PAI-1 or platelet reactivity were not collected. We did not have a control group of patients 80 years and older who did not receive endovascular treatment to provide relative estimates of treatment benefit. Finally, our sample size was too small for some trends that we observed to reach statistical significance.
Conclusions
The lower rates of favorable outcomes in patients 80 years and older with acute ischemic stroke mandate a more critical appraisal of the risk-to-benefit ratio before recommending endovascular treatment in this patient population. Imaging indicators such as diffusion-perfusion mismatch with MR imaging or volume-flow mismatch with CT perfusion scans may be of greater value in this population by helping identify the patients who are most likely to benefit from endovascular treatment.
Acknowledgments
Centocor (Horsham, Pa) provided grant support and the pharmacologic agent for some of the patients enrolled in the IA reteplase and IV abciximab (FDA IND 9180) protocol, though Centocor did not review the results of this analysis. The EKOS Corporation (Bothell, Wash) provided angiograms and clinical data for the authors’ independent review for the purposes of this analysis. They also provided the EKOS MicroLysUS infusion catheters and endovascular sonography devices for the patients enrolled in the feasibility analysis of their device, whose data were pooled for analysis in this study. EKOS did not analyze the data for this analysis.
Footnotes
Dr. Suri is supported by National Institute's of Health (NIH) grant 5K12-RR023247-03. Dr. Qureshi is supported in part by NIH's grant RO-1-NS44976-01A2 and the American Heart Association's Established Investigators Award 0840053N.
References
- Received September 20, 2008.
- Accepted after revision December 17, 2008.
- Copyright © American Society of Neuroradiology