Transient MR imaging signal-intensity changes in the thalamus, usually accompanied by signal-intensity changes elsewhere in the brain, have been previously reported in the setting of seizures.1–3 We report a case in which an MR image obtained shortly after a seizure episode demonstrated abnormal signal intensity isolated to the left thalamus on T2-weighted and fluid-attenuated inversion recovery imaging sequences.
A 28-year-old man presented 5 hours after a witnessed seizure, which was characterized by sudden loss of consciousness and jerking movements of the extremities. The seizure lasted approximately 2 minutes, and it was followed by a period of lethargy. The patient had a history of a motor vehicle crash 2 years prior as a result of which he had cervical spine fractures and short-term memory deficits. There was no history of alcohol or recreational drug use. At the time of presentation to our institution, the patient was on medications with the potential to lower seizure threshold, including bupropion, oxybutinin, and duloxetine, none of which was newly prescribed.
Within 3 hours of presentation, the patient gradually became alert and oriented, and he had no memory of the seizure. Bupropion was held as a possible contributing factor to the patient's seizure, and levetiracetam therapy was initiated for seizure prophylaxis.
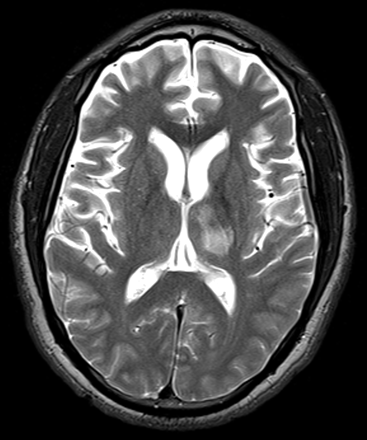
Electroencephalography (EEG), CT, and MR imaging were all performed on the day of admission. The EEG did not reveal any focal abnormality or epileptiform discharges. The CT demonstrated a nonspecific focus of hypoattenuation involving the left thalamus. The MR imaging revealed a nonenhancing T2-hyperintense focus predominantly isolated to the left thalamus (Fig. 1). There was no diffusion restriction, eliminating the possibility of cytotoxic edema from infarction. Differential diagnostic considerations included a low-grade glioma. The patient underwent a lumbar puncture, the findings of which were normal.
On day 7, a repeat MR imaging of the brain revealed complete resolution of the thalamic signal-intensity abnormality. During the remainder of the patient's admission, he remained seizure-free while on levetiracetam. On day 9, he was discharged in stable condition.
The thalamic nuclei act as relays between cortical and subcortical structures and are thought to be involved in regulation of seizure susceptibility and propagation.1 Thalamic signal-intensity abnormality, usually in conjunction with signal-intensity abnormality in other parts of the brain, has been documented by other researchers. Men et al1 reported a case with abnormal diffusion throughout the left hemispheric cortex, left thalamus, and right cerebellar hemisphere. Nagasaka et al2 reported “reversible” T2 signal-intensity abnormality involving the right temporoparietal cortex, contralateral cerebellum, and ipsilateral thalamus on an ictal MR imaging in a patient with a 1-month history of repetitive complex partial seizures. To the best of our knowledge, transient signal-intensity abnormality seen exclusively in the thalamus following a single episode of generalized seizure has not been reported previously. Our case reinforces the known role of the thalamus in seizure susceptibility and propagation. Involvement of the thalamus in our patient is strongly suggested by the development of transient thalamic signal-intensity abnormality immediately following a reported seizure.
It is not clear whether the location of the abnormal signal intensity in our patient means that the thalamus itself was the seizure focus or whether it was secondarily involved and inherently more susceptible to demonstrating signal-intensity changes. Roch et al4 studied the temporal evolution of lesions on MR imaging by using a rat epilepsy model. At 2 hours after status epilepticus, blood-brain barrier breakdown, which resolved in 6 hours, was observed only in the thalamus. It was followed by edema in the amygdala and piriform and entorhinal cortices with extensive neuronal loss. These findings resolved within 5 days.4
The rapid resolution of the signal-intensity abnormality in our patient raises the question of whether postseizure imaging should be performed more expeditiously to minimize the possibility of missing transient signal-intensity abnormality, which may help localize the seizure focus. Our case highlights the importance of follow-up imaging in a patient with abnormal imaging findings and a history of seizures before any potential medical or surgical interventions for other presumed etiologies, including a possible low-grade neoplasm.
T2-weighted MR image shows a nonenhancing hyperintense focus predominantly isolated to the left thalamus with extension to a portion of the thalamocapsular junction.
- Copyright © American Society of Neuroradiology