Abstract
BACKGROUND AND PURPOSE: Crossed cerebellar diaschisis (CCD), the decrease in blood flow and metabolism in the cerebellar hemisphere contralateral to a supratentorial stroke, is frequently reported on positron-emission tomography (PET) and single-photon emission CT (SPECT) but is rarely described with MR perfusion techniques. This study was undertaken to determine the frequency of CCD observed in acute stroke by retrospective review of a research data base of patients with acute stroke evaluated by diffusion-weighted (DWI) and dynamic contrast susceptibility perfusion MR imaging (PWI).
MATERIALS AND METHODS: PWI scans of 301 consecutive patients with acute stroke and positive DWI abnormality from a research data base were reviewed. Contralateral cerebellar hypoperfusion was identified by inspection of time-to-peak (TTP) maps for asymmetry with an absence of cerebellar abnormalities on T2-weighted scans, DWI, or disease of the vertebrobasilar system on MR angiography. In a subset of the cases, quantitative analysis of perfusion scans was performed using an arterial input function and singular value decomposition (SVD) to generate cerebral blood flow (CBF) maps.
RESULTS: A total of 47 of 301 cases (15.61%) met the criteria of CCD by asymmetry of cerebellar perfusion on TTP maps. On quantitative analysis, there was corresponding reduction of CBF by 22.75 ± 10.94% (range, 7.45% to 52.13%) of the unaffected cerebellar hemisphere).
CONCLUSIONS: MR perfusion techniques can be used to detect CCD, though the frequency presented in this series is lower than that commonly reported in the PET/SPECT literature. Nevertheless, with its role in acute stroke and noninvasive nature, MR perfusion may be a viable alternative to PET or SPECT to study the phenomenon and clinical consequences of supratentorial stroke with CCD.
Crossed cerebellar diaschisis (CCD) is a depression of neuronal metabolism and activity manifested as reduced blood flow in the cerebellar hemisphere contralateral to a supratentorial infarct, and is believed to be caused by an interruption of the corticopontocerebellar fibers.1–3 As a result of decreased afferent input, there is a decline in synaptic cerebellar Purkinje cell function, coupled with a decrease in cerebellar perfusion.3 The cerebellum retains intact vasoreactivity, and the reduction in regional metabolism in response to diminished afferent information likely causes regional arteriolar vasoconstriction. This finding is not observed on MR angiography (MRA), which can only depict changes in large vessels.3–5 Chronic CCD can cause gross structural changes of unilateral cerebellar volume loss in children and adults after a supratentorial ischemic infarct.6
Although most studies report CCD after subacute to chronic supratentorial infarction, a recent positron-emission tomography (PET) study documented CCD to be concurrent with acute unilateral supratentorial infarction, evident as early as 3 hours after symptom onset.7 It was also noted that development of CCD was related to volume of impaired perfusion but not to the severity of hypoperfusion.7 Additional studies in both humans and animals have suggested that hypoperfusion volume may not be the most critical determinant of the development of CCD, but rather the location involving the anterior middle cerebral artery territory (affecting anterior frontal and temporal lobes).3,8,9 After reperfusion with thrombolysis, CCD recovery can be seen in patients with small infarcts and is strongly associated with clinical outcome measures at all time points.7 These findings suggest that CCD may not solely be an epiphenomenon of supratentorial infarction, but also an important prognostic indicator of stroke recovery and treatment response.
Nearly all studies of CCD to date have used PET and single-photon emission CT (SPECT) to document changes in cerebral blood flow (CBF) and metabolism in the contralateral cerebellum. Perfusion-weighted MR imaging (PWI) has been shown previously to measure quantities such as relative CBF and cerebral blood volume (CBV) that are abnormal in ischemia, and which may be able to detect CCD.10 Despite its relatively common observation with PET and SPECT scanning, there has only been a single study of CCD as observed by PWI documenting diminution of CBV in the cerebellum.11 Therefore, the purpose of our study was to determine the frequency of CCD detection by PWI, by retrospective review of a research data base of patients with acute stroke who were evaluated by standard stroke protocol including diffusion-weighted (DWI) and dynamic contrast susceptibility PWI scans.
Materials and Methods
A retrospective review of an imaging data base consisting of 345 consecutive patients with stroke who consented to be part of an ongoing study of spatial neglect and aphasia was performed. Our Institutional Review Board approved this study. All 345 patients (183 with dominant hemisphere and 162 with nondominant hemisphere involvement) presented to our institution between January 2001 and May 2006 and underwent an MR imaging scan that included DWI and PWI within 5 days of symptom onset. Among them, 301 patients had abnormal hyperintense signal intensity on DWI. Review of the clinical history and imaging results was conducted by J.T.K. and A.W.L., and identification of positive CCD cases was performed by D.D.M.L. A positive CCD case had to fulfill the following: any patient with a clinical history of a focal neurologic deficit from an ischemic vascular cause and evaluated with MR imaging within 5 days of symptom onset, with evidence of a unilateral supratentorial DWI lesion, and an accompanying decrease in perfusion on PWI on the basis of increased time-to-peak (TTP) of the contralateral cerebellar hemisphere. In addition, the perfusion deficits should not be accompanied (in the same hemisphere) by any abnormality in MRA of the vertebrobasilar system, or concomitant cerebellar pathologies present on DWI and conventional T2-weighted (including fluid-attenuated inversion recovery) MR imaging scans. Some patients were further evaluated with digital subtraction cerebral angiography, contrast-enhanced MRA of the neck, or both; these scans, when available, were also examined to exclude possible posterior fossa vascular disease.
Image Acquisition and Processing
All studies were performed on a 1.5T Signa scanner (GE Healthcare, Milwaukee, Wis) with use of the standard quadrature transmit-receive head coil. In addition to 3D time-of-flight MRA (TR, 38 msec; TE, 4.6 msec; flip angle [FA], 20°; section thickness, 1 mm; matrix, 320 × 224; FOV, 22 × 22 cm), conventional T1-weighted (TR, 300–500 msec; TE, 8–15 msec; FA, 90°; section thickness, 5 mm; matrix, 256 × 192; FOV, 24 × 24 cm) and T2-weighted (TR, 3800–4500 msec; TE, 90–108 msec; FA, 90°, echo-train length, 20; section thickness, 5 mm; matrix, 256 × 192; FOV, 24 × 24 cm), fluid-attenuated inversion recovery (TR, 8800 msec; TE, 133 msec; FA, 90°; inversion time [T1], 2200 msec; section thickness, 5 mm; matrix, 256 × 192; FOV, 24 × 24 cm), and isotropic echo-planar DWI were obtained (bmax = 1000 s/mm2; TR, 10,000 msec; TE, 120 msec). Echo-planar PWI images were recorded during bolus injection of 0.1 mmol/kg of gadopentate dimeglumine (Gd-DTPA) at 5 mL/sec by using single-shot gradient-echo echo-planar imaging (TR, 2000 msec; TE, 60 msec; 30 dynamics). PWI scans were postprocessed to generate maps of TTP.
We computed volumes of infarct on DWI by using the program ImageJ (Imaging processing and analysis in JAVA, NIH, rsb.info.nih.gov/ij) for all sections that displayed a signal intensity abnormality by manual segmentation. The same program was used to calculate the volumes of supratentorial hypoperfusion on TTP maps, segmented manually after setting a threshold value of TTP at 4 seconds or longer.12–14 Cases of cerebellar hypoperfusion contralateral to supratentorial infarct and hypoperfusion were identified by visual inspection of the TTP maps without a set threshold. A subset of these positive cases (n = 20) were further evaluated by a quantitative analysis program, “Penguin,”15–17 with an arterial input function chosen automatically at the level of the circle of Willis, and single-value decomposition, to calculate the mean CBF of each cerebellar hemisphere by drawing a region of interest encompassing the entire hemisphere. This was performed in 20 patients, in whom perfusion raw data were available for quantitative analysis. Differences of TTP (defined as TTPcontralateral − TTPipsilateral in the cerebellum and TTPipsilateral − TTPcontralateral in the cerebrum), as well as the degree of hypoperfusion (defined as [CBFipsilateral − CBFcontralateral]/CBFipsilateral × 100%] in the cerebellum and [CBFcontralateral − CBFipsilateral]/CBFcontralateral × 100% in the cerebrum) were calculated. Volumes of cerebellar hypoperfusion were not measured because, to the best of our knowledge, there is no literature on an appropriate TTP threshold for CCD.
Statistical Analysis
Means of continuous variables from the CCD− and CCD+ groups were compared by a 2-tailed t test assuming unequal variances. Relationship between variables was analyzed by the Pearson correlation test. The significance level for all tests was P < .05.
Results
A total of 51 of the 301 patients were identified to demonstrate asymmetric cerebellar hypoperfusion, 4 of whom were excluded because of underlying vertebrobasilar disease on the same side or evidence of concomitant cerebellar infarction confounding the cause of cerebellar hypoperfusion. The remaining 47 (15.61%) patients were deemed to fulfill the criteria for CCD. Note that 5 cases with vertebrobasilar disease were included as CCD because the pattern of hypoperfusion could not be explained by the vascular abnormality (eg, in the ipsilateral cerebellum). None of the 47 patients with CCD showed cerebellar signs of dysfunction. Table 1 summarizes the age of the patients, volumes of DWI, and supratentorial TTP abnormalities in both CCD− and CCD+ populations. There is no significant difference between the mean age of the 2 groups of patients (CCD− mean age, 61 years; CCD+ mean age, 60 years; P = .67, t test). The mean volume of the DWI abnormality is significantly higher in the CCD+ cases (55.57 cm3 compared with 19.76 cm3 in CCD− cases; P = .0001, t test). In a similar fashion, the mean volume of supratentorial TTP abnormality is significantly higher in the CCD+ cases (58.79 cm3 compared with 29.42 cm3 in CCD− cases; P = .004, t test).
Comparison between CCD− and CCD+ cases in patient age, DWI volume, and volume of abnormal TTP in the supratentorial ischemic lesion*
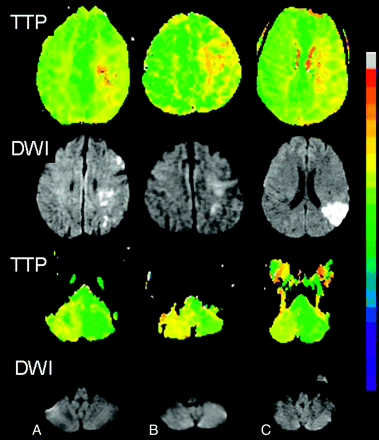
Figure 1 illustrates 3 selected cases of CCD, demonstrating a similar pattern of supratentorial DWI abnormality (acute infarct in cases A and C, subacute infarct in case B; all 3 with dominant hemisphere involvement) without evidence of infratentorial ischemia. At the same section level, PWI showed prolonged TTP supratentorially accompanying the contralateral cerebellar hypoperfusion. In case A, the patient was 70-year-old man with hypertension who presented with sudden right-hand weakness, numbness, and slurred speech. MR imaging was performed at 7 hours after initial onset of symptoms. DWI showed multiple small areas of acute watershed infarcts in the left cerebral hemisphere (DWI volume, 18.24 cm3), with a large mismatched perfusion deficit in the left middle cerebral artery (MCA) territory (TTP volume, 30.51 cm3). Case B was a 74-year-old Filipino man with history of hypertension, diabetes, coronary artery disease, and renal failure requiring hemodialysis, who presented with severe Broca aphasia after a hypotensive episode following right eye enucleation for glaucoma. Despite the acute onset of symptoms, MR imaging performed within 2 days showed only a subacute left MCA territory infarct (small foci of faint DWI hyperintensity without corresponding hypointensity on the apparent diffusion coefficient map; DWI volume, 3.44 cm3; TTP volume, 73.52 cm3) with abrupt, marked stenosis at the left mid M1 segment and absent flow-related enhancement in the distal left MCA branches. In case C, a 47-year-old African American man with hypertension and diabetes, presented with sudden onset of Wernicke aphasia and paraphasia. MR imaging on the same day (approximately 12 hours after symptom onset) showed acute infarct in the left parietal and posterior temporal lobes and insula (DWI volume, 29.68 cm3; TTP volume, 26.97 cm3). MRA demonstrated stenoses in the M1 and M2 regions of the left MCA, which were later confirmed by conventional angiography.
Three cases (A, B, and C) of infarct affecting the dominant cerebral hemisphere associated with contralateral cerebellar hypoperfusion. PWI maps according to TTP show supratentorial and contralateral cerebellar hypoperfusion relative to the opposite hemisphere (first and third rows). DWI (second and fourth rows) shows acute infarct co-localized with supratentorial perfusion abnormality. The co-localized cerebellum (fourth row) shows no evidence of infarct in the region of the hypoperfusion.
A wide range of DWI and TTP volumes of the supratentorial infarcts was observed: in the 47 positive CCD cases (Table 1), the volume of supratentorial DWI lesion ranged from 1.78 cm3 to 258.40 cm3, and TTP abnormality ranged from 0 to 307.52 cm3 (in these cases, supratentorial TTP abnormality may be calculated as low as 0 because of the threshold set at TTP ≥ 4 seconds, and in some cases, there was reperfusion). Of note, in 2 cases a small volume (2.18 and 14.8 cm3, respectively) of focal DWI abnormality was localized to the thalamus. There were also 2 cases (not included in the positive CCD cases) in which there was unilateral supratentorial perfusion deficit without any DWI lesion, and a contralateral cerebellar hypoperfusion was also observed. Because our inclusion criteria for CCD required the presence of a supratentorial DWI lesion, these 2 cases were excluded.
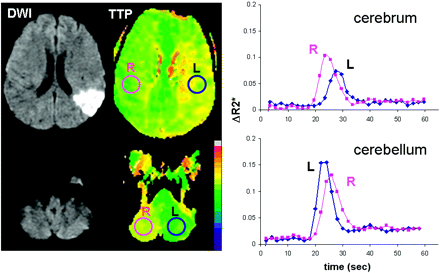
When regions of interest were selected from the cerebrum and cerebellum, plots of ΔR2* versus time clearly showed dispersion of the curves of abnormally perfused tissue relative to the contralateral side (Fig 2), both in the cerebellum as well as in the supratentorial infarct.
ΔR2* (= −ln(S/S0)/TE) versus time curves of case C from Fig 1 in the cerebrum and cerebellum show a shift of bolus arrival time to the right side (ie, prolonged TTP) in the hypoperfused brain with acute infarct, and in the contralateral cerebellum without evidence of ischemia.
The perfusion raw data were available in 20 of 47 cases, and these were further analyzed with the “Penguin” software to produce TTP and CBF measurements in the cerebral and cerebellar hemispheres (Table 2). In each case, cerebellar hypoperfusion was observed in the cerebellum (without any imaging abnormalities on DWI) contralateral to the cerebral infarct (Fig 1). TTP tended to be higher in the supratentorial ischemic zone (4.98 ± 3.10 sec) compared with the contralateral cerebellum (2.02 ± 1.29 sec; P = .001, t test). There was, however, a substantial reduction of CBF both supratentorially (31.33% ± 17.71%) and infratentorially (22.75% ± 10.94%; P = .07, t test). No statistically significant changes in CBV were observed between the 2 hemispheres; the overall mean in this cohort showed a 6% CBV reduction (with a large SD) compared with the ipsilateral cerebellum (Table 2).
Summary of differences in TTP and degree of CBF and CBV reduction of the affected hemisphere relative to the unaffected hemisphere in the cerebellum and cerebrum in CCD+ cases (n = 20)*†
There was a weak correlation between DWI and TTP volumes of r = 0.33 (Pearson correlation) that was statistically significant (P < .0001) in the supratentorial ischemic infarct when all 301 cases were included, with a similar r = 0.35 in analyzable CCD+ cases (n = 20) that did not reach statistical significance (P = .06). Supratentorial TTP values showed a weak correlation with DWI volume (r = 0.32, P = .097) and with TTP volume (r = 0.26, P = .15) of the ischemic lesion, but without statistical significance. No significant correlation was found between the supratentorial DWI volume and cerebellar TTP change, between the supratentorial TTP volume and cerebellar TTP change, or between supratentorial DWI or supratentorial TTP volume and CBF reduction, either in the cerebrum or in the cerebellum.
Discussion
The main finding of our study was that PWI, with use of the bolus-tracking technique, can be used to depict asymmetric cerebellar hypoperfusion contralateral to an acute supratentorial ischemic infarct, a condition commonly termed CCD in the PET/SPECT literature. This hypoperfusion was clearly illustrated on TTP maps and was confirmed to be a result of reduced blood flow by quantitative CBF analysis techniques. The flow reduction in the contralateral cerebellar hemisphere was unexplained by vascular disease in the posterior circulation, supporting the notion of deafferentation involving anatomically distinct, but functionally linked, brain regions, rather than a primary ischemic event in the cerebellum. Despite clear evidence of cerebellar hypoperfusion, no patients in this series showed any neurologic deficits attributable to cerebellar dysfunction.
In the one previous study11 on MR imaging detection of CCD, it was found that cerebellar CBV was reduced; however, our study did not reproduce this result. This may be for several reasons; the patient population in the previous study was all in the subacute to chronic stages of stroke (range, 6–120 days), whereas our study consisted of patients within 5 days of symptom onset. Furthermore, the previous study involved several methodologic differences, including lower temporal resolution (3 sec), thicker sections (10 mm), and lack of quantitative analysis by use of arterial input function deconvolution.
Although CCD is predominantly reported after large MCA infarcts, there is disagreement in the literature relating to the severity of CCD and infarct volume.18,19 Our study suggests that infarct volume contributes to the development of CCD because there was significantly larger DWI volume in CCD+ cases; however, the sample size (n = 20) was too small to establish a correlation between the severity of CCD and DWI abnormality. A spectrum of infarct volumes resulted in CCD, including 2 cases of small infarcts both involving the thalamus, which is an important “relay station” between the cortical and cerebellar pathways. This observation is consistent with other groups’ findings of the location of the infarct being an important factor for CCD (eg, case reports of CCD correlating with a variety of thalamic pathologic conditions, including hematoma and infarction).18,20,21 A PET study of acute stroke showed significant correlation between CCD and supratentorial hypoperfusion volume,7 which is also supported in this study, in that there is a significantly greater abnormal TTP (and DWI) volume in the CCD+ cases. However, again, no significant correlation was found between cerebellar CBF reductions and supratentorial TTP (or DWI) volumes (r = −0.118; P = .33; n = 20). The small sample size in this series also precluded a meaningful correlation with the location of hypoperfusion or infarct.
Subacute function (and 1-month recovery) has been shown to be worse in patients with stroke and CCD compared with those without CCD, though a confounding factor was that infarct volumes were also generally larger in the CCD group.22 Therefore, although no reports to date show that cerebellar hypoperfusion from CCD is associated with permanent infarction, the fact that chronic deafferentation results in measurable structural abnormalities suggests that CCD is not an altogether benign process.6 A serial PET study with longitudinal follow-up at multiple time points after thrombolytic therapy also highlighted the usefulness of CCD as an indicator of clinical outcomes.7
The incidence of CCD in acute stroke is unclear; however, several series of mixed stroke chronicity in the nuclear medicine literature have reported frequencies of greater than 50%.11,23,24 Given the relatively common observation of CCD on PET and SPECT, it is surprising that the MR imaging literature is almost devoid of this observation, and the detection rate (15.61%) in our series is substantially lower compared with these previous PET/SPECT studies. This low incidence could be from multiple factors, but a major reason may be the relative insensitivity of TTP to detect mild hypoperfusion, particularly if performed at relatively low temporal resolution. Cases with subtle cerebellar TTP prolongation may have been missed by visual inspection (qualitative analysis). Other reasons that CCD may not have been observed in previous PWI studies include the common use of perfusion protocols that did not have whole-brain (ie, cerebellar) coverage, or failure to generate TTP maps of the posterior fossa. Inclusion and exclusion criteria, and hence patient populations, may be different between previous PWI and PET/SPECT studies.
It is conceivable that the reported incidence of CCD on PET/SPECT may, in some cases, be overestimated. For instance, in studies that did not also include MR imaging, it is possible that patients with small supratentorial infarcts were missed (if only evaluated with CT and nuclear medicine modalities, or even with conventional MR imaging without diffusion-weighted sequences), or patients with underlying posterior circulation vascular disease were inadvertently included. Both of these factors would lead to the increased apparent incidence of CCD in the acute stroke population. In our study, it was strictly required that there be an absence of any structural, DWI, and angiographic abnormalities in the cerebellum that may account for the observed perfusion abnormality. To illustrate, 53 (17.61%) of 301 patients in our sample population had a 5 mL or less infarct volume on DWI, and their condition would have likely been excluded as nonstroke or transient ischemic attack in previous CT-based studies.
As can be expected from this patient population with multiple risk factors for stroke, underlying atherosclerotic cerebrovascular disease is prevalent. Vertebrobasilar disease can often be found concomitantly with anterior circulation pathologic conditions. Among a sample of 51 patients initially identified with asymmetric cerebellar hypoperfusion in this series, 9 (17.64%) had evidence of vertebrobasilar disease (on the basis of MRA) or previous cerebellar infarct (on the basis of T2-weighted scans); 4 (7.84%) patients were excluded because the possibility of these pathologic conditions accounting for cerebellar hypoperfusion could not be ruled out completely.
Conclusions
Crossed cerebellar diaschisis, or reduced perfusion in the cerebellum contralateral to acute cerebral infarction, can be detected with PWI as demonstrated by prolonged TTP and diminished CBF. The lower detection rate of CCD (15.61%) in our series compared with the PET/SPECT literature may be related to multiple factors, including differences in patient selection criteria. PWI is nevertheless an attractive alternative to PET because it is less complicated, is less expensive, does not involve radiation, and has higher spatial resolution. It is also more widely available and can be combined with structural and vascular MR techniques within a single session. Therefore, PWI seems to be a suitable tool for the future study of the clinical significance of supratentorial stroke with CCD, although, at present, the prognostic value of CCD as observed by PWI is unknown.
Acknowledgments
We give special thanks to Cameron Davis and Melissa Newhart for technical support. We also thank Dr. Leif Ostergaard for the “Penguin” analysis software.
Footnotes
This research was supported by NIH RO1 NS R01047691, P41RR15241, K12 RR017627, and the Willbur Memorial Cerebrovascular Research Fund.
Doris D.M. Lin, MD, who is affiliated with the Johns Hopkins University School of Medicine, Baltimore, conducted the statistical analysis.
References
- Received August 11, 2008.
- Accepted after revision October 27, 2008.
- Copyright © American Society of Neuroradiology