Abstract
BACKGROUND AND PURPOSE: External beam radiation therapy (XRT) for head and neck cancer is known to induce hypothyroidism and cause morphologic changes in the thyroid gland. This retrospective study investigates change in the size of the thyroid gland detectable by CT after XRT for laryngeal cancer.
MATERIALS AND METHODS: The measured width of the thyroid lobes in 61 patients treated nonsurgically with XRT for laryngeal cancer between 2000 and 2003 on posttherapy CT was compared with that on pretherapy CT. Absolute and percentage changes in measured thyroid width following XRT were analyzed according to chemotherapy administration and posttherapy thyroid function.
RESULTS: Eighty-five percent (52/61) of patients had a decrease in the width of the thyroid gland. The average change in width measuring −4.7 mm and −13.8% (SD, 5.7 mm and 19.9%) occurred at an average of 758 days following completion of XRT (mean, 402-1534 days) and was significant (P = .002). Average change in width between hypothyroid patients (n = 19, −6.1 mm and −20.0% change) and euthyroid patients (n = 42, −4.1 mm and −11.1% change) was not significant (P = .20 absolute change and P = .11 percentage change). The average change in width between patients receiving chemotherapy (n = 31, −5.5 mm and −16.1% change) and patients not receiving chemotherapy (n = 30, −3.9 mm and −11.5% change) was not significant (P = .26 absolute change and P = .37 for percentage change).
CONCLUSIONS: Most nonsurgical patients receiving XRT for laryngeal cancer have a significant decrease in the width of their thyroid glands detected on CT. The average change in the size of the thyroid gland does not differ when development of hypothyroidism or chemotherapy administration are considered.
External beam radiation therapy (XRT) is an integral part of treatment for head and neck cancer. Clinicians providing posttherapy care for these patients recognize that changes can occur in the thyroid gland after XRT to the head and neck leading to hypothyroidism. Hypothyroidism is documented in approximately 20%–67% of patients receiving XRT for Hodgkin or non-Hodgkin lymphoma and approximately 20%–31% of patients receiving XRT for head and neck cancers in the first 5 years after treatment.1–12 Hypothyroidism typically occurs within 5 years after completing XRT. Morphologic changes to the thyroid gland after XRT, such as the development of solid or cystic masses, are well documented by sonography and histology.13–18 The thyroid gland is known to decrease in the size after XRT as demonstrated by sonography.18
Patients with laryngeal cancer are followed after treatment with CT of the neck to evaluate treatment response and monitor for recurrence; however, radiologists rarely mention changes in the size of the thyroid gland on follow-up CT reports following XRT for head and neck cancer. We have occasionally reported a decrease in the size of the thyroid gland in these posttherapy patients. We asked the following questions: How does the thyroid gland change in the size following XRT on CT, does the administration of neoadjuvant or adjuvant chemotherapy affect the change in the size of the thyroid gland following XRT, and does the change in the size of the thyroid gland differ between patients who develop hypothyroidism and those who remain euthyroid during the follow-up period?
Materials and Methods
Two hundred forty-five patients receiving XRT for laryngeal cancer between June 2000 and January 2003 at our cancer center were considered for this study. Institutional review board approval was obtained, and patient information was protected according to the Health Insurance Portability and Accountability Act standards. Operative notes, clinical notes, laboratory data, and neck CT studies were collected from the electronic medical records. Patients undergoing total laryngectomy, partial laryngectomy, cervical lymph node dissection, or tracheostomy as part of the initial treatment for their laryngeal cancer were excluded. Patients who did not complete their full prescribed dose of radiation therapy were excluded. Patients who did not have pre-XRT or post-XRT CT scans of the neck available for review or who had extension of their primary tumor into the thyroid gland were excluded. Patients with a diagnosis of a thyroid disorder before therapy for laryngeal cancer were excluded. Patients who did not have specific laboratory documentation of thyroid function or a clinical note addressing thyroid function at follow-up visits were excluded.
The age of each patient, the date of completion of the prescribed dose of XRT, and the use of neoadjuvant or adjuvant chemotherapy were collected from the electronic medical records.
Contrast-enhanced CT of the neck was performed on a 4-detector LightSpeed Plus CT scanner (GE Healthcare, Milwaukee, Wis). Scanning was performed at 5-mm section thickness from the top of the orbits to the hard palate with the gantry angled in the plane of the skull base and 2.5-mm section thickness through the neck from the midramus of the mandible through the thoracic inlet with the gantry angled in the plane of the larynx. Patients received 65-mL iohexol (Omnipaque; GE Healthcare) at 1.5 mL/s with a 50-second delay in scanning followed immediately by 35-mL iohexol at 1 mL/s. Scanning began at the thoracic inlet and proceeded cranially. Images were reconstructed to 1.25-mm section thickness for viewing in head and neck soft-tissue window settings (width 300 HU and level 70 HU) on PACS workstations.
A diagnostic radiology resident (M.M.M.-T.) and a senior neuroradiologist (A.J.K.) performed the thyroid measurements by using the electronic measurement calipers on a PACS workstation. They were not blinded to the clinical data collected from the patients’ charts. A single axial CT section through the widest part of the thyroid gland was chosen, and the greatest width of each lobe of the thyroid gland excluding the isthmus was measured to the nearest tenth of a millimeter on the pre-XRT neck CT and compared with the same axial section on each post-XRT CT. The time after therapy when each post-XRT CT was acquired was recorded. If the patient underwent delayed surgery for persistent or recurrent disease, data were not collected from subsequent CTs.
The thyroid stimulating hormone (TSH) levels were retrieved from the electronic medical records system when available. The normal range for TSH at the cancer center laboratory is 0.5–5.5 mIU/L. TSH values >5.5 mIU/L were recorded as elevated and a sign of thyroid gland failure. Free T4 levels were not routinely obtained in all patients and were, therefore, not recorded for the purpose of this study. The follow-up clinic notes were reviewed, and note was made of a clinician placing the patient on thyroid hormone replacement therapy as a sign of thyroid gland failure. Statements based on physical examination findings and patient symptoms in the follow-up clinic notes indicating normal thyroid gland function were recorded as positive indicators of a euthyroid state.
The absolute change and the percentage change in the summed width of the 2 lobes of the thyroid gland compared with the pre-XRT CT image were calculated for each post-XRT CT measurement. The measurements were grouped into quarter years on the basis of the interval the scanning was performed after XRT completion, and the average and SD for the absolute and percentage change in thyroid gland width were calculated. Linear regression was applied to the average absolute change and the average percentage change for measurements acquired in each quarter year to detect a trend in the change of thyroid gland size with time. Patients were then subdivided within each quarter-year group into those who developed hypothyroidism by the end of the study and those who remained euthyroid throughout the study.
A 2-tailed t test was used to detect differences in the absolute change in size and the percentage change in size of the thyroid gland within each quarter year. A 2-tailed paired t test was applied to the pre-XRT and the latest post-XRT measurements of thyroid gland width for each patient to determine if there was a significant change in thyroid gland size after XRT. A 2-tailed t test was used to detect differences in the absolute change in size and the percentage change in size of the thyroid gland in patients who received chemotherapy compared with those who did not and in patients who developed hypothyroidism during the follow-up period compared with those who did not. Finally, patients were placed into 4 categories on the basis of administration of chemotherapy and development of hypothyroidism during the follow-up period, and the percentage change and absolute change in thyroid gland width calculated from the latest post-XRT CT of each group were compared by using the Kruskal-Wallis test.
Results
Two hundred forty-five patients received XRT for laryngeal cancer during the study period. Eighty-six patients were excluded because they did not have pre-XRT and at least 1 year of post-XRT CT of the neck performed at our institution. Seventy-five patients were excluded because they had surgical intervention, either a total or partial laryngectomy, neck dissection, or tracheostomy before completing at least 1 year of post-XRT CT follow-up imaging. Three patients were excluded because they did not complete their prescribed dose of radiation therapy. Three patients were excluded because they had a pretreatment diagnosis of hypothyroidism, and 17 patients were excluded because there was no laboratory data or clinical note documenting thyroid function at follow-up visits. Sixty-one patients fulfilled the criteria for inclusion in the study. There were 56 cases of squamous cell carcinoma, 1 case of carcinoid, 1 case of rhabdomyosarcoma, 1 case of synovial sarcoma, and 2 cases of small cell cancers of the larynx. The average patient age was 57.3 years with a range from 20 to 80 years. Thirty-one patients received chemotherapy as part of their treatment, and 30 did not. The average total time for CT follow-up of these patients was 758 days after completing XRT, with a range from 402 to 1534 days.
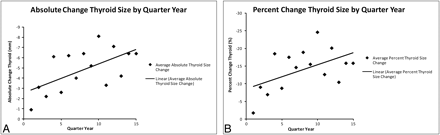
Figure 1 shows the graphs with best-fit lines for average absolute and percentage change in size of the thyroid gland on follow-up CTs of all patients grouped by quarter year since completion of XRT. Eighty-five percent (52/61) of patients had a measured decrease in size of their thyroid gland, and 15% (9/61) of patients had a measured increase in size of their thyroid gland on follow-up CT. Figure 2 is an example of a patient who had a decrease in size of the thyroid gland after radiation therapy. The average change in thyroid gland size measured on the last follow-up CT for each patient was −4.7 mm and −13.8% (SD, 5.7 mm and 19.9%). The change in width of the thyroid gland after XRT as measured on the last follow-up CT was statistically significant according to the 2-tailed paired t test (P = .002).
Graphs comparing average absolute change in thyroid gland size (A) and average percentage change in thyroid gland size (B) after XRT by quarter-year increments. Linear regression lines show trends toward greater average decrease in size of the thyroid gland on CT performed later after completion of XRT.
Contrast-enhanced axial CT images of the neck through the level of the thyroid gland. A, Preradiation therapy. B, Six months after completing radiation therapy, there is a decrease in the size of the thyroid gland. C, Four years after completing radiation therapy, there is continued decrease in the size of the thyroid gland.
The average change in the size of the thyroid gland measured on the final CT study was −6.1 mm and −20.0% (SD, 6.8 mm and 24.3%) for patients developing hypothyroidism during the follow-up period and −4.1 mm and −11.1% (SD, 5.1 mm and 17.2%) for patients remaining euthyroid during the follow-up period. The differences in absolute change and percentage change in the size of the gland between the hypothyroid and euthyroid groups were not statistically significant using a 2-tailed t test (P = .20 for absolute change in size and P = .11 for percentage change in size).
The average change in size of the thyroid gland measured on the final CT study was −5.5 mm and −16.1% (SD, 6.4 mm and 22.3%) for patients receiving chemotherapy and −3.9 mm and −11.5% (SD, 4.8 mm and 17.2%) for patients not receiving chemotherapy. The differences in absolute change and percentage change in the size of the gland between the chemotherapy and no chemotherapy groups were not statistically significant using a 2-tailed t test (P = .26 for absolute change in size and P = .37 for percentage change in size).
We compared the absolute and percentage change in size of the thyroid gland following XRT, taking into consideration the functional status of the thyroid gland and the use of chemotherapy, with the Kruskal-Wallis test, which did not show a statistically significant difference in absolute or percentage change in size of the thyroid gland (H = 4.68 with 3 df yielding a P value of .20 for difference in absolute change in size and H = 7.10 with 3 df yielding a P value of .07 for percentage change in size).
Discussion
The biochemical changes to the thyroid gland function caused by XRT to the head and neck are well documented in the clinical literature.1–12 XRT causes microvascular and parenchymal damage to the gland, which results in decreased function several years after completing XRT. Eighty-five percent (52/61) of the patients included in this study demonstrated a measured decrease in the width of the thyroid gland on follow-up CT, with measurable changes occurring in several patients within the first quarter year following completion of XRT. The wide range in the absolute and percentage change in the width of the gland suggests that the causes are likely multifactorial, including radiation-induced vascular damage, parenchymal cell damage, autoimmune-mediated damage, and fibrosis of the capsule preventing compensatory hypertrophy of the gland.2 Fifteen percent (9/61) of patients included in the study had a measured increase in the size of the thyroid gland on follow-up CT. Again, this is likely multifactorial, including enlargement resulting from vascular, parenchymal, and autoimmune-mediated damage as well as compensatory hypertrophy of the gland as hormonal feedback systems stimulate the functionally damaged gland.
The data presented in Fig 1 show a progression in average thyroid gland size decrease as time from XRT therapy increases. These averages by quarter year include all patients regardless of whether they had an increase or a decrease in the size of the gland, showing that the phenomena that cause a measureable decrease in the size of the gland predominate over those that cause enlargement of the gland during the study period in this population of patients. This study demonstrates that the average size of the thyroid gland decreases after XRT for laryngeal cancer on follow-up CT, and the result is statistically significant but does not demonstrate a statistically significant difference in thyroid gland size after XRT for patients who developed hypothyroidism or patients who received chemotherapy compared with those who did not. The difference in percentage change in the size of the thyroid gland in euthyroid-versus-hypothyroid patients approaches statistical significance with a 2-tailed t test yielding a P value of 0.11. Similarly, the difference in percentage change in the size of the thyroid gland when both thyroid functional status and administration of chemotherapy were considered approaches statistical significance with a Kruskal-Wallis test yielding a P value of .07.
Thirty-one percent (19/61) had biochemical failure of the thyroid gland within the study period, which is at the upper limit of hypothyroidism rates following XRT for head and neck cancer published in the clinical literature, despite the fact that the literature states that it takes up to 5 years to develop hypothyroidism after XRT to the neck.1–12 The percentage of patients developing hypothyroidism during the study period is likely exaggerated because lack of clinical data on thyroid function was an exclusion criterion for the study. A disproportionate number of euthyroid patients were probably excluded because it may be easier to document positive signs and symptoms of a disorder rather than negative signs and symptoms. Thyroid function was likely not tested or reported for all patients because of the subspecialty referral and follow-up patterns in our cancer center, with many patients receiving follow-up care outside the center. In addition, the average follow-up time of patients in this study was 758 days—just over 2 years. Previous studies have shown that biochemical failure of the gland after XRT can take up to 5 years to develop, and more patients may have gone on to develop thyroid gland failure if followed for a longer time period.
Imaging follow-up and thyroid function testing intervals were not standardized in this retrospective study. There was a bias toward patients with higher stages of disease at presentation because these patients with a higher stage were more likely to be followed by CT for a year or more after XRT than patients with stage I laryngeal cancer, who were more likely to be followed by clinical examination and laryngoscopy. These patients with a higher stage may have received a higher radiation dose delivered to the thyroid gland because of the inclusion of nodal chains in the low neck in the radiation port. This study did not estimate radiation dose to the thyroid gland.
Although anecdotal evidence of a visually detected change in the size of the thyroid gland in post-XRT patients motivated this study, we did not rigorously test the measurement thresholds for visual detection of thyroid gland size change by axial CT. We do not intend for radiologists to report the thyroid gland width on every post-XRT follow-up neck CT; rather, we seek to make the imaging community aware that posttherapy CT can demonstrate a change in the size of the thyroid gland.
Conclusions
In summary, the thyroid gland changes in the size after XRT for laryngeal cancer, with most patients demonstrating a decrease in the size of the gland and a minority of patients demonstrating an increase in the size of the gland. This study does not demonstrate a statistically significant average change in the size of the thyroid gland on follow-up CT scans after XRT between patients who develop hypothyroidism and patients who remain euthyroid or between patients who receive chemotherapy and those who do not during the time course of follow-up for this study.
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Head and Neck Radiology, September 21, 2005; San Francisco, Calif.
References
- Received August 16, 2007.
- Accepted after revision October 17, 2008.
- Copyright © American Society of Neuroradiology