Abstract
SUMMARY: Unlike the more widely reported gradient-echo echo-planar perfusion-weighted imaging (EPI-PWI) technique, spin-echo (SE) EPI relative cerebral blood volume maps select for blood volume in microvessels <8 μm in diameter. This first report of SE-EPI PWI for distinguishing brain metastasis from high-grade glioma demonstrated 88% sensitivity and 72% specificity in 83 patients. We discuss differences in microvessel architecture between high-grade glioma and brain metastasis that may explain the surprising success of SE-EPI in this application and may deserve further investigation.
Distinction of brain metastasis from high-grade glioma by MR imaging remains an important unsolved clinical problem. Percentage recovery analysis of time-intensity curves from gradient-echo echo-planar (GE-EPI) dynamic susceptibility contrast perfusion-weighted imaging (PWI)1 has been proposed to distinguish these tumor types by assessing increased capillary permeability in brain metastasis, but to our knowledge, no published report yet documents the accuracy of this method. Reports from the pathology literature suggest that in addition to permeability, brain metastasis and high-grade glioma neovessels differ substantially in vascular architecture,2–7 and in particular, metastases may contain a substantially larger proportion of intermediate-sized dysplastic vessels up to 70 μm in diameter.8–12 Recent literature demonstrates that spin-echo EPI (SE-EPI) PWI has a substantially different vessel-size sensitivity profile from GE-EPI PWI.13–15 Because SE-EPI PWI relative cerebral blood volume (rCBV) maps reflect nearly exclusively the blood volume of microvessels <8 μm in diameter, in contrast to the admixture of microvessels and larger vessels assessed with GE-EPI, we hypothesized that SE-EPI rCBV maps would allow reliable distinction of brain metastasis from high-grade glioma. To our knowledge, this is the first report using SE-EPI PWI to make this distinction.
Technique
Forty-three subjects with high-grade glioma (20 cases of glioblastoma multiforme, 14 anaplastic astrocytomas, 7 anaplastic oligoastrocytomas, and 2 unspecified high-grade gliomas) and 40 patients with brain metastasis (2 sarcomas; 38 carcinomas, including 20 lung, 5 melanoma, 1 basal cell, 4 breast, 1 esophageal, 2 colorectal, 1 prostate; and 4 adenocarcinomas of unknown primary) were included chronologically from our institutional data base. Ninety-eight percent (42) of patients with high-grade glioma and 68% (27) of patients with brain metastasis had received previous chemotherapy; 63% (27) of patients with high-grade glioma and 85% of patients with metastasis (34) had received radiation (Table 1). Following institutional protocol, SE-EPI PWI (TE = 80 ms, TR = 1900–2216.7 ms, section thickness = 10 mm, matrix = 1282–2562) was performed by using a 4-mL/s bolus injection of 30-mL double-dose gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, NJ) (0.15–0.3 mmol/kg depending on body weight) and was coregistered with delayed postgadolinium T1-weighted imaging. Imaging was performed on 1.5T or 3T commercial MR imaging scanners (GE Healthcare, Milwaukee, Wis), and PWI data were processed by using a perfusion-processing software package (GADW 4.3 FuncTool software, GE Healthcare), which creates color rCBV maps by calculating the change in transverse relaxivity (R2) from signal-intensity measurements, integrates the area under the relaxivity curve and corrects for TE, according to the following standard formula: 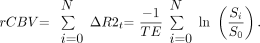
Patient Demographics
Following previously published “hot spot” methods, we selected a roughly 100-mm2 region of interest (ROI) within the region of maximal rCBV and recorded the average rCBV within the ROI as the rCBVmax.16,17 We calculated a normalized rCBV (nCBV) by dividing rCBVmax by the rCBV of an equivalent ROI in the contralateral normal-appearing white matter16 and performed percentage recovery analysis in the same ROI according to previously reported GE-EPI techniques.1
Results
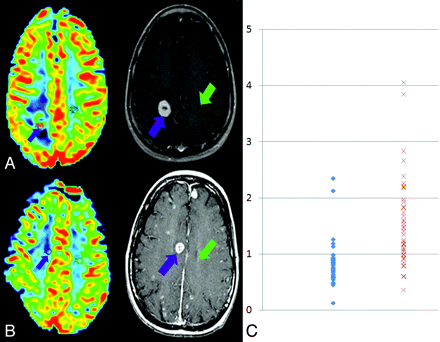
The average nCBV within enhancing high-grade glioma was 1.53 ± 0.79 (0.59–4.05) compared with 0.82 ± 0.40 for brain metastasis (0.48–2.12), and differences between nCBV averages and ranges for the 2 groups were significant (P < .05) (Fig 1C). Sensitivity and specificity for detection of brain metastasis were 95% and 51%, respectively, by using an nCBV threshold of 1.3; and 36% and 93%, by using a 0.7 threshold. The optimal threshold occurred at 1.0, where sensitivity and specificity were 88% and 72% (Table 2).
A and B, rCBV maps (left) demonstrate increased blood volume in the enhancing high-grade glioma (A) and decreased blood volume in the enhancing brain metastasis (B). Corresponding T1-weighted images are shown (center) with black arrows representing the location of ROI selection in the lesion and white arrows indicating control ROI location in normal appearing contralateral white matter placement. C, Scatterplot of the nCBV measurements in brain metastasis (blue diamonds) and high-grade glioma (red squares). (Color figure is available on-line only at www.ajnr.org.)
Diagnostic Accuracy of SE-EPI nCBV vs. Percent Recovery Using Optimal Thresholds of nCBV = 1.0 and % recovery = 66%
Percentage recovery analysis yielded average signal-intensity recoveries of 0.86 for high-grade glioma and 0.75 for brain metastasis (P < .05). Percentage recovery analysis sensitivity and specificity to distinguish brain metastasis from high-grade glioma were 18% and 93% by using a previously reported1 signal-intensity recovery threshold of 0.66. Optimal accuracy, obtained by using a signal-intensity recovery threshold of 0.85, yielded a sensitivity and specificity of 84% and 48% (Table 2).
Discussion
In this retrospective series, SE-EPI-derived rCBV maps allowed reliable distinction of brain metastasis from high-grade glioma and were superior to percentage recovery analysis for distinguishing brain metastasis from high-grade glioma by using SE-EPI (Fig 1). Because of the limitations regarding the retrospective design of this initial report, further study is required before this finding can be applied clinically. Most important, although both groups received radiation and chemotherapy, differences in treatment response between the groups could conceivably contribute to the differences observed.
Although definitive testing of our hypothesis that the success of SE-EPI is related to differences in vessel-size distribution between brain metastasis and high-grade glioma is beyond the scope of this report, a number of limitations of the current data deserve consideration in the design of future studies. First, as our percentage recovery analysis demonstrates, there is a significant difference between the brain metastasis and high-grade glioma groups in microvessel permeability. Future studies designed to investigate whether the observed effect is actually related to vessel-size differences between brain metastasis and high-grade glioma will require more rigorous correction for T1 shortening effects due to first-pass capillary leak.18 Additionally, the use of interleaved GE-SE vessel-size imaging5,6 may be of interest. Finally, because of the paucity of direct analyses of metastatic vessel-size distribution in the pathology literature, correlation with microscopic imaging would be valuable.
Conclusion
SE-EPI-derived rCBV maps promise to assist in accurate preoperative distinction of brain metastasis from high-grade glioma. If more rigorous analysis in treatment-naïve patients and prospective investigation support the preliminary findings presented here, SE-EPI PWI may prove an important clinical tool.
Footnotes
Both authors contributed equally to this work.
References
- Received April 10, 2008.
- Accepted after revision June 5, 2008.
- Copyright © American Society of Neuroradiology