Abstract
BACKGROUND AND PURPOSE: Mild cognitive impairment (MCI) represents a transitional state between normal aging and Alzheimer disease (AD). Our goal was to determine if specific temporoparietal regions can predict the time to progress from MCI to AD.
MATERIALS AND METHODS: MR images from 129 individuals with MCI were analyzed to identify the volume of 14 neocortical and 2 non-neocortical brain regions, comprising the temporal and parietal lobes. In addition, 3 neuropsychological test scores were included to determine whether they would provide independent information. After a mean follow-up time of 5 years, 44 of these individuals had progressed to a diagnosis of AD.
RESULTS: Cox proportional hazards models demonstrated significant effects for 6 MR imaging regions with the greatest differences being the following: the entorhinal cortex (hazard ratio [HR] = 0.54, P < .001), inferior parietal lobule (hazard ratio [HR] = 0.64, P < .005), and middle temporal gyrus (HR = 0.64, P < .004), indicating decreased risk with larger volumes. A multivariable model showed that a combination of the entorhinal cortex (HR = 0.60, P < .001) and the inferior parietal lobule (HR = 0.62, P < .01) was the best predictor of time to progress to AD. A multivariable model reiterated the importance of including both MR imaging and neuropsychological variables in the final model.
CONCLUSIONS: These findings reaffirm the importance of the entorhinal cortex and present evidence for the importance of the inferior parietal lobule as a predictor of time to progress from MCI to AD. The inclusion of neuropsychological performance in the final model continues to highlight the importance of using these measures in a complementary fashion.
Individuals classified with mild cognitive impairment (MCI) experience memory loss to a greater extent than expected for age, and they progress to a diagnosis of Alzheimer disease (AD) at a faster rate than controls.1 It has been hypothesized that MCI represents a transitional phase between normal function and AD2 for many individuals. A number of MR imaging studies of subjects with MCI have demonstrated that selected brain regions within the medial temporal lobe, particularly the hippocampus and entorhinal cortex, are reduced in volume in subjects with MCI in comparison with control subjects. Subjects with MCI who show such reductions are at increased risk for progression to AD (for a review of this topic see Atiya et al,3 Chetelat and Baron,4 Kantarci and Jack,5 and Anderson et al6). These findings are consistent with neuropathologic reports that have demonstrated that the entorhinal cortex and hippocampus have considerable neuropathology early in the course of AD.7–9
Fewer MR imaging studies have been conducted examining the role of additional temporoparietal regions (beyond the entorhinal cortex and hippocampus) in the earliest stages of AD. Of these studies, some have manually drawn regions of interest within temporoparietal regions, such as the fusiform and superior temporal gyrus.10,11 In addition, others have used whole-brain measures, such as voxel-based morphometry,12–14 fluid registration methods,15,16 and cortical thickness approaches,17,18 thus avoiding the need to measure individual anatomic areas. Taken together, the results of these studies provide evidence that areas within the parietal and lateral temporal lobes may additionally be involved in the earliest stages of AD. However, it remains unclear which specific regions or which combination of these regions beyond the medial temporal region best predicts progression of disease from MCI to AD.
The present study was undertaken to examine which temporoparietal regions best predict progression from MCI to AD. Here, we examined 14 neocortical and 2 non-neocortical regions of interest on MR images, comprising the temporal and parietal lobes, obtained from 129 individuals with MCI, who were subsequently followed with time. After a mean follow-up interval of 5.0 years, 44 of these individuals had progressed to a diagnosis of AD. It was therefore possible, by using Cox proportional hazard models, to determine which specific temporoparietal regions, alone or in combination, could be used to predict time to progress from MCI to a diagnosis of AD. Neuropsychological measures of episodic memory and executive function were included for these subjects to test whether the inclusion of these measures in the models provided predictive information beyond the MR imaging measures.
Materials and Methods
Selection of Participants
A total of 129 individuals were included in this study. They were recruited through the print media (rather than from a clinical or other medical referral source). The advertisements for subjects indicated that a research study was seeking individuals with and without memory difficulty.
Potential subjects underwent a multistage screening procedure. The details of the screening procedures have been described elsewhere.19 Briefly, to be included in the study, participants had to be 65 years of age and older; have an informant who could provide information about their daily function; be free of significant underlying medical, neurologic, or psychiatric illness; and be willing to participate in the study procedures. In addition, individuals with evidence of major vascular risk factors (eg, atrial fibrillation, insulin-dependent diabetes mellitus, cerebral infarcts, etc) were excluded. The subjects in the present study were selected because they were mildly impaired but nondemented and had a Clinical Dementia Rating (CDR) score20 of CDR = 0.5.
The study procedures also included a medical evaluation (consisting of a physical examination and medical history, electrocardiogram, and standard laboratory tests), a semistructured interview, neuropsychological testing, an MR imaging scan, a single-photon emission CT scan, and blood withdrawn for genetic analysis. All subjects provided informed consent before the initiation of the study, in accordance with the requirements of the Human Research Committee of Massachusetts General Hospital (Boston, Mass).
Assessment of Clinical Severity
The degree of clinical severity of the subjects was evaluated by an annual semistructured interview. This interview generated both an overall CDR rating and a measure known as the CDR sum of boxes (CDR-SB).21 The interview was based on the initial subject protocol that was used in the development of the CDR scale20 and included a set of questions regarding functional status asked of the subject and a collateral source (eg, family member, friend), along with a standardized neurologic, psychiatric, and mental status evaluation of the subject. The mental status evaluation included the following: the Blessed Memory and Orientation Test,22 which assessed episodic memory, working memory, and orientation; a set of similarities and differences, which assessed executive function; calculations that assessed arithmetic skill and general knowledge; and a standardized language evaluation, including naming, repetition, and comprehension. To be sensitive to clinical impairments at the mildest end of the spectrum, we added a special set of questions to the interview, and the reliability and validity of the revised interview were examined.23 The mean inter-rater reliability of the CDR ratings in the context of the present study was high (r = 0.99, P < .0001), as was the inter-rater reliability of the 6 CDR subcategories (r = 0.90) that were used to generate the overall CDR rating.23 The CDR-SB represents the sum of the ratings in each of the 6 CDR subcategories, thus the inter-rater reliability for this measure was also high.
In the current study, each interview was administered by a masters or doctoral level clinician (eg, psychiatrist, neuropsychologist, or physician assistant) and was performed without knowledge of the other study procedures, including the MR imaging findings. The interview took approximately 1–2 hours to complete. A consensus review of each subject was conducted annually by 2 or more members of the research group (which included the interviewers mentioned above).
Group Characteristics at Baseline and at Follow-Up
Baseline.
A total of 129 mildly impaired individuals, with a mean CDR-SB score of 1.3 (SD = 0.8) and a range of 0.5–3.5, were examined in this study. Table 1 shows the mean age, educational status, Mini-Mental State Examination (MMSE)24 scores, sex distribution, and apolipoprotein E (APOE) status of the subjects. In general, the subjects were well educated and had high scores on the MMSE.
Descriptive statistical information for the subjects in the study*
The distribution of CDR-SB scores among the mildly impaired subjects was broad (Table 1). At the mild end of the spectrum (ie, CDR-SB = 0.5–1.5), many subjects would not meet psychometric cutoffs commonly used to select subjects with MCI in epidemiologic studies and clinical trials.25,26 The subjects at the more impaired end of the spectrum (ie, CDR-SB ≥ 2) were comparable with subjects with MCI recruited from these settings on the basis of the likelihood of progression to a diagnosis of AD.23 We use MCI here to refer to the entire group of mildly impaired subjects. A retrospective review of the cases indicated that approximately two thirds would fall into the category of amnestic MCI, whereas approximately one third would be considered nonamnestic MCI cases, based on the revised criteria for MCI.27
Follow-Up.
Of the 129 individuals who were mildly impaired at baseline, 44 subsequently received a clinical diagnosis of AD (mean follow-up time, 5.0 ± 3.6 years), whereas 85 remained mildly impaired (mean follow-up time, 6.9 ± 4.4 years). Of those who remained mildly impaired at follow-up, 54 had CDR-SB scores that declined but their impairments had not progressed to the point where they received a diagnosis of AD, 28 had CDR-SB scores that remained stable, and 3 had CDR-SB scores that increased. Approximately 19% of these mildly impaired subjects (n = 16) had a CDR-SB of ≥2, and approximately 81% (n = 69) had a CDR-SB score of 0.5–1.5.
Diagnosis of Dementia on Follow-Up.
As part of the annual review of each case, the consensus diagnostic process determined the following: 1) whether the individual had sufficient impairment for a diagnosis of dementia, and if so, 2) whether the dementia was consistent with research criteria for AD28 or another known diagnostic entity (eg, frontotemporal dementia or vascular dementia).29,30 Diagnoses were based on findings from a combination of clinical history, medical records, laboratory evaluation, and neuroimaging studies (eg, the presence of cerebral infarcts). Only subjects with a diagnosis of probable AD on follow-up were included in the outcome group presented here.
MR Image Acquisition
The MR images used in this study were acquired on a 1.5T Signa scanner (GE Healthcare, Milwaukee, Wis). T1-weighted 3D spoiled gradient-recalled echo (SPGR) scans were acquired by using the following sequence: 1 coronal acquisition, TR = 35 ms, TE = 5 ms, FOV = 220 mm, flip angle = 45°, section thickness = 1.5 mm, matrix size = 256 × 256, NEX = 1.
Regions of Interest
The MR images obtained at baseline were processed by using the FreeSurfer software package (http://surfer.nmr.mgh.harvard.edu).31,32 First, each scan was normalized for spatial intensity changes by using the N3 algorithm, followed by an intensity normalization procedure.32 Next, the skull was removed by using a skull-stripping algorithm.33 The images were then segmented to identify the dorsal, ventral, and lateral extent of the gray/white matter boundary, to provide a surface representation of the cerebral white matter.31,32 The quality of the skull stripping and the accuracy of the gray/white matter tissue boundary for each subject were reviewed by an anatomically knowledgeable operator (R.S.D.) and edited, as needed, to produce an anatomically accurate surface representation of the cortical white matter (ie, to ensure the exclusion of bone and other non-neocortical matter from white matter, and to fill in artifactual “holes” in the white matter surface that were inconsistent with known neuroanatomy).
Once the white matter representation was complete, an automatic topology-correction algorithm was applied that corrects for small topologic defects (ie, voxel misclassifications that result in erroneous “bridges” or “connections” between areas in the white matter).34 The topologically corrected white matter surface was then used in a deformation algorithm that identified the neocortical (ie, gray matter) surface of the brain.35 Last, the white and gray matter surfaces were visually inspected and further edited, as needed, for anatomic accuracy, by a trained operator (R.S.D.) (ie, to ensure the exclusion of skull from gray matter and the proper outward deformation of the white matter).
The neocortex of the brain on the MR images was then automatically subdivided into 32 gyral-based regions of interest (in each hemisphere). To accomplish this, we used a registration procedure that aligns the cortical folding patterns36 and probabilistically assigns every point on the cortical surface to 1 of the 32 regions of interest.37 The regions of interest generated were examined for anatomic accuracy and edited by an anatomically knowledgeable operator (R.S.D.), as needed, to ensure that they adhered to previously published boundary definitions.37 For the purposes of this study, we focused on the 14 regions of interest that corresponded to neocortical regions from the temporal and parietal cortices, because pathologic evidence of AD is primarily in the temporal and parietal regions early in the course of disease. The regions selected included the following: 1): the banks of the superior temporal sulcus, 2) entorhinal cortex, 3) fusiform gyrus, 4) inferior parietal lobule, 5) inferior temporal gyrus, 6) isthmus of cingulate cortex (ie, the caudal portion of the posterior cingulate), 7) posterior cingulate cortex (ie, the rostral portion of the posterior cingulate), 8) middle temporal gyrus, 9) parahippocampal gyrus, 10) precuneus cortex, 11) superior parietal lobule, 12) superior temporal gyrus, 13) supramarginal gyrus, and 14) temporal pole.
The non-neocortical regions of the brain were subdivided into 19 regions of interest (in each hemisphere). As with the neocortical regions, an algorithm automatically assigned each voxel in this portion of the scan to 1 of 19 neuroanatomic regions of interest.38 These regions of interest were edited by an anatomically knowledgeable operator (R.J.K.), as needed, to ensure that they adhered to previously published boundary definitions.39,40 Because pathologic evidence of AD is primarily in the temporal and parietal regions early in the course of disease, for the purposes of the current study, we selected 2 of the 19 non-neocortical regions of interest corresponding to regions in the temporal lobe1: the amygdala and2 hippocampus.
In total, 16 neocortical and non-neocortical temporoparietal regions of interest were used in this study. Figure 1 depicts the location of 1 of these regions of interest, namely the inferior parietal lobule. For all of the analyses performed here, the volumes of the right and left hemispheres for each individual region of interest were added together. Editing of the regions of interest was necessary because SPGR scans have lower contrast to noise than do the specific sequences on which the image analysis algorithms used here were optimized. Z-scores were computed for each region of interest on the basis of the distributions of volumes found in the sample.
Illustration of the location of the inferior parietal and medial temporal regions of the brain on a 3D image on 1 hemisphere of the brain. Please see Fig 5 from Fischl et al, 200238 and Fig 1 from Desikan et al, 200637 for a detailed color depiction of all 16 temporoparietal regions of interest used in this study.
Neuropsychologic Measures
As part of participation in this study, all subjects were also administered a neuropsychological battery that was independent of the assessment of clinical severity. The composition of the entire battery has been previously described.19 Three test scores from this battery were selected for analysis in the present study because they had previously been shown to be sensitive predictors of time to progression from MCI to AD.41 These 3 tests included the following: 2 tests of episodic memory: the total number of words learned across the 4 learning trials of the California Verbal Learning Test (CVLT)42 and the total number of words learned across the 4 learning trials of the Selective Reminding Test (SRT)43; and an executive function test: the time to complete Part B of the Trail Making Test (Trails B).44 All neuropsychological measures were standardized to have zero mean and unit variance, averaged over the combined study sample, to facilitate interpretable coefficients in the Cox proportional hazards models and facilitate comparisons of effect sizes across tests. Before standardization, the raw time to complete Trails B was log-transformed so that the distribution was more normal.
Statistical Analysis of Data
The time-to-progression data were analyzed by using Cox proportional hazards models as implemented in the PHREG procedure in SAS, Version 8 (SAS Institute, Cary, NC). These models tested whether specific predictors (ie, z-scores based on MR imaging measures from temporoparietal regions) are associated with time to a diagnosis of AD. The hazard ratio (HR) indicates the differential risk per 1 unit difference in the predictor. For instance, if the HR is 1.06 for the volume of the entorhinal cortex, each 1-SD decrease increases the risk by 6%; or if the HR is 0.57 for the entorhinal cortex, each 1-SD decrease in the volume of this region increases the risk by 43%.
The primary focus of the analyses was time from study entry to the end point of interest (ie, the diagnosis of AD). A set of univariate and multivariable Cox models was performed. The initial set of analyses included 2 bivariate (single predictor) models and 1 multivariable (multiple predictor) model. The 2 bivariate models were as follows1: The first bivariate model for each of the MR imaging measures was “crude” in that it only included an adjustment for intracranial cavity (ICC) size.2 The second bivariate model for each of the MR imaging measures was adjusted for both ICC and age. The multivariable model, which was designed to be the “best” multivariable Cox model (given the set of variables), was then developed as follows: It began with the inclusion of ICC and age, which were “forced” into the model (ie, these 2 variables were entered into the model and were retained even if they were not significant). The 16 z-scores based on MR imaging measures were then added but were only retained if they were significant at the .05 level. Age was controlled for linearly. Subsequent analyses repeated these models by using binary variables based on the z-scores for each region of interest, with those with z-scores smaller than 1 SD below the mean placed in 1 group and those with larger volumes placed in a comparison group. In these analyses, HRs >1 indicated increased risk of progression to AD with region-of-interest volumes below the 1 SD cutoff. The models were also repeated with the hippocampus forced into the models.
A separate set of analyses involved recalculation of the models with the inclusion of the 3 neuropsychological measures. These variables were added to determine whether the inclusion of neuropsychological data provided redundant or additive information to the MR imaging data concerning prediction of progression. In addition to these proportional hazards analyses, Spearman rank correlation coefficients were used to examine the relationship between the 16 temporoparietal MR imaging volumes and the 3 neuropsychological measures.
The proportional hazards assumption was evaluated descriptively by checking whether the negative log of survival probabilities associated with higher levels of each covariate was constant multiples of those of the lower levels across the entire range of event time. This approach was extended to the multivariate Cox model by examining such patterns across the levels of each linear predictor. Model fit was also examined descriptively by checking the distribution of the martingale residuals, as well as deviance residuals.45
Results
MR Imaging Volumetric Temporoparietal Measures and Time to Diagnosis of AD
Bivariate Cox models were first constructed to assess the likelihood of time to progression to a diagnosis of AD for each of the 16 temporoparietal MR imaging measures, by using only an adjustment for ICC. Of the 16 variables, 7 were statistically significant at the P < .05 level or greater (on-line Table 1). A second set of bivariate Cox models was then completed that included an adjustment for both ICC and age (on-line Table 1). Of the 16 variables, 6 of the same 7 variables were statistically significant at the P < .05 level or greater. The only variable that was significant in the first univariate model but was not significant in the second univariate model was the hippocampus. There were large effects for the entorhinal cortex (HR = 0.54 [0.37–0.78], P < .001), the inferior parietal lobule (HR = 0.64 [0.46–0.88], P < .005), and the middle temporal gyrus (HR = 0.64 [0.47–0.86], P < .004). As anticipated, the level of statistical significance was decreased by the inclusion of the age adjustment.
Identifying the Best Temporoparietal Predictors of Time to a Diagnosis of AD Using a Multivariable Model
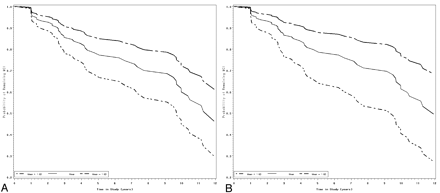
A multivariable model of predictors of time to progression to diagnosis of AD among subjects with MCI at baseline was then constructed. This model examined which z-scores, in combination, were the best predictors of time to diagnosis. In this model, only the entorhinal cortex (HR = 0.57 [0.39–0.83], P < .004) and the inferior parietal lobule (HR = 0.68 [0.47–.98], P < .05) were statistically significant. Figure 2 shows survival curves for time to progress from MCI to a diagnosis of AD, as a function of the volume of the inferior parietal lobule.
Survival curves for prediction of time to progression from MCI to a diagnosis of AD (based on the adjusted univariate model [shown at the mean, and 1 SD above and below the mean]), as a function of variation in the MR imaging volume of the inferior parietal lobule (A) and the entorhinal cortex (B).
Assessment of Uniformity of Risk in Time to Diagnosis of AD
Several analyses were performed to further examine the uniformity of risk for time to a diagnosis of AD based on the 2 temporoparietal MR imaging variables that were significant in the multivariable model (ie, the entorhinal cortex and the inferior parietal lobule). First, the multivariable model was recalculated, with the volume of the hippocampus forced into the model. The presence of the hippocampus did not reduce the HR of the entorhinal cortex (HR = 0.57 [0.27–0.94], P < .005) and slightly reduced the HR of the inferior parietal lobule (HR = 0.68 [0.48–99], P < .04).
Second, by using binary variables based on the z-scores for each region of interest, on the basis of the set of 129 subjects with MCI, we computed an additional set of Cox regressions. Among these binary variables, we found that 17 subjects had z-scores for volume of the entorhinal cortex that were 1 SD below the mean and 24 subjects fell into this category on the basis of the z-score for the volume of the inferior parietal lobule. The results of these additional Cox models showed a substantial increase in the HR for both of the variables in the model: the entorhinal cortex (HR = 4.75 [2.27–9.94], P < .0001) and the inferior parietal lobule (HR = 2.33 [1.16–4.69], P < .01). This model was then recalculated with the binary variable based on the z-score for the hippocampus forced into the analysis. The results showed minimal change in the HR for both variables in the model: the entorhinal cortex (HR = 4.27 [1.91–9.54], P < .0004) and the inferior parietal lobule (HR = 2.65 [1.29–5.47], P < .008).
Contribution of Neuropsychologic Variables to the Bivariate and Multivariable Models and Correlations with MR Imaging Volumes
Further analyses were performed to assess whether the addition of specific neuropsychologic variables, previously shown to be significant predictors of time to progress from MCI to AD,41 would provide additional predictive information, above and beyond the MR imaging measures, when added to the bivariate and multivariate models. First, the bivariate models were recalculated, with the inclusion of 4 additional variables: years of education and the 3 neuropsychological test scores (ie, the CVLT, the SRT, and Trails B). The same 6 z-scores for MR imaging variables identified above were still statistically significant at the P < .05 level or greater, after the addition of these 4 variables. The HRs for each of the 6 MR imaging variables from the bivariate analyses were similar in magnitude to those observed in the models in which neuropsychological variables had not been included. Significant effects were observed for the entorhinal cortex (HR = 0.56 [0.40–0.81], P < .01), amygdala (HR = 0.60 [0.41–0.88], P < .01), inferior parietal lobule (HR = 0.61 [0.44–0.87], P < .01), supramarginal gyrus (HR = 0.61 [0.42–0.87], P < .01), middle temporal gyrus (HR = 0.63 [0.45–0.89], P < .01), and the fusiform gyrus (HR = 0.68 [0.49–0.94], P < .05).
Second, the same 4 variables (ie, years of education and the 3 neuropsychological test scores) were added to the multivariable model to determine whether any of them would be selected instead of the MR imaging variables as the best predictors of progression from MCI to AD. In this multivariable model, only the entorhinal cortex (HR = 0.63 [0.44–0.91], P < .01), and Trails B (HR = 2.75 [1.17–6.65], P < .05) were statistically significant. The inferior parietal lobule (HR = 0.70 [0.48–1.02], P = .06) demonstrated a trend toward statistical significance.
For the correlations between the MR imaging volumes and CVLT, the parahippocampal gyrus (r = 0.25, P = .005) and temporal pole (r = 0.20, P = .05) demonstrated a significant relationship, with the hippocampus (r = 0.15, P = .09) demonstrating a trend toward statistical significance. With SRT, the parahippocampal gyrus (r = 0.21, P = .01), temporal pole (r = 0.23, P = .01), and hippocampus (r = 0.31, P = .001) demonstrated a significant relationship. With Trails B, none of the regions of interest demonstrated a significant relationship.
Discussion
These findings reaffirm the importance of an MR imaging measure of the entorhinal cortex as a predictor of progression from MCI to a diagnosis of AD. In every analysis that was performed, the volume of the entorhinal cortex was a better predictor of progression from MCI to AD than any of the other 15 temporoparietal MR imaging measures. These data are in agreement with a number of previous reports that have concluded that the volume of entorhinal cortex is better at predicting the likelihood of progression from MCI to AD than that of the hippocampus.46–48
These results also emphasize the value of a volumetric measure of the inferior parietal lobule. This measure, when used in combination with the entorhinal cortex, was the best predictor of time to progress from MCI to AD. Moreover, it remained statistically significant even when the volume of the hippocampus was forced into the model. Prior studies by using fluid-registration, cortical thickness, and voxel-based morphometry have implicated areas within the lateral parietal cortex to be involved in the earliest stages of AD and to be a predictor of progression,12–18 but this is the first volumetric study, to our knowledge, to demonstrate the relative importance specifically of the inferior parietal lobule in predicting progression in comparison with the many other brain regions within the temporal and parietal lobes.
This finding is consistent with pathologic studies of AD showing that specific laminae in the inferior parietal lobule are preferentially affected in the early stages of the disease.49,50 Moreover, projections from the inferior parietal lobule target several subfields within the medial temporal lobe,51–53 suggesting that atrophy in the inferior parietal lobule likely reflects the spread of AD pathology from the temporal lobe to an interconnected region in the parietal lobe.
The analyses presented here also demonstrate that MR imaging volumetric measures may be useful in identifying the subset of subjects with MCI who are at a particularly high risk of progression to AD. Those subjects with MCI whose entorhinal cortex and inferior parietal lobule volumes were 1 SD below the mean for the group as a whole at baseline had markedly increased risk of progression to AD compared with those whose volumetric measures did not fall ≥1 SD below the mean. It is increasingly recognized that subjects with MCI from a community volunteer−based cohort generally include a broad range of severity. The present findings suggest that it should be possible to use MR imaging measures, independent of clinical and neuropsychological measures, to identify the subset of subjects with MCI at greatest risk for progression.
These findings also suggest that MR imaging volumetric data provide information concerning time to progress from MCI to AD that is independent of neuropsychological measures that have previously been shown to be significant predictors of progression. A number of temporoparietal regions, including the entorhinal cortex and inferior parietal lobule, continued to predict significantly time to progression, even after the addition of the neuropsychological variables to the bivariate models. Moreover, the entorhinal cortex was retained as one of the best predictors of conversion in the multivariable model that also included a neuropsychological variable, suggesting that MR imaging and neuropsychological data may provide complimentary information in relation to prediction of progression from MCI to AD. This finding differs somewhat from a recent report suggesting that once neuropsychological measures are considered, the added value of MR imaging measures is small.47 The difference between the findings reported here and the previous study may be related to the fact that the earlier study examined subjects who were more mildly impaired than those examined in the present study and additionally did not include a test of executive function, such as that included here.
Correlations between tests of episodic memory function (CVLT and SRT) and volumes of the parahippocampal gyrus, temporal pole, and hippocampus are consistent with the fact that these temporal lobe regions are critical for normal memory function (for a discussion of this topic see Blacker et al41). Of interest, Trails B, a test of executive function, did not demonstrate any significant correlations with any of the temporoparietal regions but was one of the best predictors in the multivariable model when combined with the MR imaging volumes. This suggests that regions beyond the temporal and parietal lobes are potentially responsible for executive function and may additionally be significant predictors of progression.
A concern in this study pertains to the difference in APOE-ε4 between the 2 groups. Because more MCI-converters were APOE-ε4 positive than the MCI-nonconverters and the ε4 allele of this gene is overrepresented in patients with AD compared with the general population,54 1 possibility is that the presence of APOE-ε4 alone can best account for the time to progress from MCI to AD. Prior work from our research group has demonstrated that the influence of the ε4 allele on the time to progress from MCI to AD is largely accounted for by neuropsychological measures and assessments of clinical severity,41 thus disputing the notion that the presence of this allele can solely account for the time to progress from MCI to AD.
The present study has several strengths. The subjects were followed prospectively and then categorized, after their symptoms had evolved, by clinicians with no access to the MR imaging data. The image analysis methods presented here permit a comparison of the relative strengths of prediction for each anatomic region within the temporal and parietal lobe and can be combined with survival analyses to determine which individual or combination of regions of interest best predicts time to progress from MCI to AD.
One limitation of this study is that only brain regions within the temporal and parietal cortices were examined. It is, therefore, possible that regions elsewhere in the brain may also be significantly related to time to progression from MCI to AD. In addition, a longer follow-up interval may have resulted in a larger number of subjects progressing to AD; as a result, other regions of interest, in addition to the ones presented here, may have been identified as significant predictors of time to progress from MCI to AD.
Conclusions
Taken together, these findings suggest the importance of examining brain regions not emphasized in previous MR imaging studies, such as the inferior parietal lobule, a region selected as one of the best predictors of time to progress from MCI to AD. These MR imaging measures may also be useful in identifying individuals at particularly high risk for progression and could readily be used for selecting subjects for clinical trials in MCI or for guiding treatment decisions, when improved medications become available.
Acknowledgments
We thank Dr Mary Hyde for invaluable assistance with data analysis and Drs Svetlana Egorova, Amanda Dow, and Marisa Tricarico for assistance with data management.
Footnotes
This work was supported by grants from the National Institute on Aging (P01-AG04953), the National Center for Research Resources (P41-RR14075, R01-RR16594, U24-RR021382), the National Institute for Biomedical Imaging and Bioengineering (R01-EB001550), and the National Institute for Neurological Diseases and Stroke.
indicates article with supplemental on-line table.
References
- Received August 5, 2008.
- Accepted after revision October 4, 2008.
- Copyright © American Society of Neuroradiology