Abstract
SUMMARY: Chikungunya, an alphavirus presenting with fever, rash, and polyarthritis, is derived from the Makonde word that means “that which bends up,” in reference to the crippling manifestations of the disease. Most often it is a self-limiting febrile illness. Neurologic complications of Chikungunya infection have been reported. We are reporting the clinical and neuroimaging data in 2 patients with Chikungunya encephalomyeloradiculitis and brain autopsy findings in 1 patient.
Chikungunya virus was first isolated in Calcutta, India, in 1963,1 with several reported outbreaks in India since then. The first isolation of the disease worldwide was in 1952, following an outbreak on the Makonde Plateau. The symptoms include fever, headache, rash, and severe arthralgia. Many of these symptoms are indistinguishable from dengue fever, and simultaneous isolation of both dengue and Chikungunya from sera of patients has been reported.2 Chikungunya virus, an Old World alphavirus, is related antigenically to O’nyong-nyong virus and is not known to be neurotropic. However, meningoencephalitis has been reported in outbreaks in India and the Reunion Islands.3,4 We present the clinical, neuroimaging, and brain autopsy findings of Chikungunya encephalomyeloradiculitis, a relatively unknown and rare complication of the infection.
Case Reports
Case 1
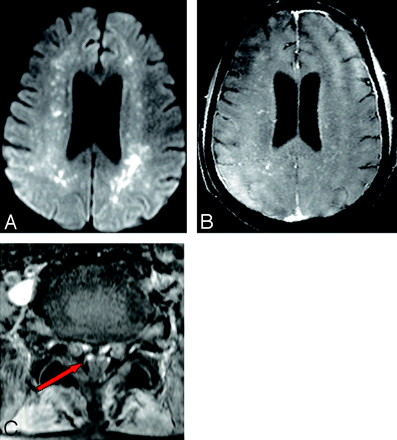
A 65-year-old man had low-grade fever and joint pain, which were treated with analgesics. A few days later he became drowsy. Findings of a complete blood analysis were normal. However, the patient's condition deteriorated clinically. On examination, he was semiconscious with neck rigidity, no limb movements, and an extensor plantar response. CSF analysis revealed elevated proteins, low sugars, and a few cells with lymphocyte predominance. CSF and serum were positive for immunoglobulin M (IgM) antibodies to Chikungunya virus. Intravenous methylprednisolone was administered for 5 days with 5 cycles of plasmapheresis. The patient was shifted to our hospital 45 days after the onset of symptoms. Electromyography (EMG) and nerve-conduction studies revealed acute generalized motor axonal neuropathy with no sensory component. Brain MR imaging revealed bilateral frontoparietal white matter lesions with restricted diffusion (Fig 1A), which enhanced on postcontrast T1-weighted (T1WI) fat-saturated images (Fig 1B). Spinal MR imaging revealed enhancement of the ventral cauda equina nerve roots (Fig 1C). He was administered IV dexamethasone and given supportive treatment; however, he did not improve clinically and was lost to further follow-up.
A, Bilateral frontoparietal white matter lesions with restricted diffusion. B, Postcontrast enhancement on T1WI fat-saturated images and mild ventriculomegaly. C, Postcontrast T1WI fat-saturated axial images at the L4 level reveal enhancement of ventral nerve roots (arrow).
Case 2
A 73-year-old man had fever and joint pain, which were treated with analgesics. A few days later, he became drowsy and unresponsive with reduced limb movements. He was admitted 1 week after initial onset of symptoms. Examination revealed absent deep tendon reflexes with an extensor plantar response. CSF studies revealed increased cells (predominantly lymphocytes), elevated proteins, and low sugar. CSF and serum were positive for IgM antibodies to the Chikungunya virus. Electroencephalography revealed bilateral nonspecific slowing with progressive decline in cerebral output on repeat studies. EMG revealed generalized sensorimotor peripheral neuropathy. Brain MR imaging showed bilateral frontoparietal white matter lesions with restricted diffusion and no enhancement. Spinal MR imaging revealed enhancement of the ventral cauda equina nerve roots. Intravenous dexamethasone and supportive treatment were given, but his clinical condition deteriorated and he died.
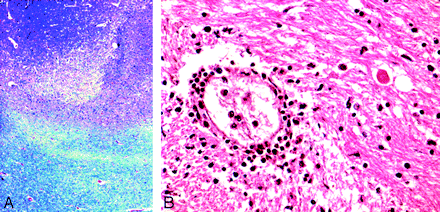
A brain autopsy was performed. On gross examination, the brain was swollen and showed subarachnoid cerebellar hemorrhages. All sections revealed significant edema with focal ischemic changes in the frontal and occipital cortexes and the internal capsule. Small foci of demyelination were detected in the subcortical white matter (Fig 2A). There was evidence of white matter edema with myelin pallor. Sparse microglial response was noted in the cortical gray matter and diencephalic area, though microglial nodule formation and neuronophagia were not evident. A few regions of perivascular lymphocytic infiltrates and gitter cells were noted in the basal ganglia (Fig 2B). Viral inclusion bodies and reactive astrocytosis on glial fibrillary acidic protein staining were conspicuous by their absence.
A, Foci of demyelination in subcortical white matter (Luxol Fast Blue stain, 20×). B, Perivascular lymphocytic basal ganglia infiltrates (H&E stain, 20×).
Discussion
Chikungunya virus belongs to the genome of alphaviruses and causes an acute viral infection characterized by fever, rash, and arthralgia.5 The Aedes mosquito is the principal vector, and the virus is maintained by transmission to nonhuman primates. The incubation period ranges from 1 to 12 days. Severe arthritic involvement is common in adults, whereas children occasionally present with seizures. Both patients were infected during the recent epidemic in an endemic zone of eastern Maharashtra, India.
The neuroimaging findings were bilateral frontoparietal white matter lesions with restricted diffusion, which is described as an early sign of viral encephalitis.6-9 Ali et al10 reported restricted diffusion in 7 of 14 patients infected with West Nile virus. Restricted diffusion precedes signal abnormalities seen on fluid-attenuated inversion recovery (FLAIR) images and is also known to resolve earlier than the FLAIR signal abnormalities during the recovery period. Both patients had restricted diffusion with FLAIR hyperintensities and poor clinical outcomes. Bilateral white matter lesions with restricted diffusion also occur in vasculitis or acute demyelination. Viral infections do not induce perivascular demyelination except in progressive multifocal leukoencephalopathy. The presence of perivascular lymphocytic infiltrates with demyelinating foci is nonspecific and is common to viral infections, indicating sensitization to viral antigen and microglial activation. In case 1, enhancement of multifocal bilateral frontoparietal white matter nodular lesions was noted. However, Ali et al10 reported no enhancement in lesions with restricted diffusion in cases of West Nile virus fever. Nodular perivascular enhancement also occurs in lymphocytic vasculitis, lymphoma, and acute demyelination.
Enhancing cauda equina nerve roots are reported in patients with the West Nile virus fever.10 Similar enhancement is seen in Guillain-Barré syndrome (GBS), tuberculous arachnoiditis, and subarachnoid spread of tumor. Enhancing cauda equina and cranial nerves are known in GBS; however, periventricular enhancing white matter lesions with restricted diffusion are not seen. Clumping of enhancing nerve roots is seen in tuberculous arachnoiditis. Nodular and thick enhancement of nerve roots is seen in subarachnoid tumor spread.
Microscopy revealed perivascular lymphocytic infiltrates, predominantly in the basal ganglia. Neuropathologic studies in West Nile encephalitis have noted similar perivascular mononuclear infiltrates.10 Gitter cell reaction in the basal ganglia may be a response to ischemic or demyelinating lesions. Absence of the astroglial response is especially seen in Arbovirus infections by dengue and Japanese encephalitis. No definite viral inclusions or microglial nodules with neuronophagia were seen which is unlike Japanese encephalitis. These findings are nonspecific, and no definite histologic conclusion could be drawn from them. Restricted diffusion is secondary to cytotoxic edema and may be secondary to plasma leakage from capillaries and venules associated with immune-mediated allergic perivascular demyelination.
Reasons for re-emergence of Chikungunya in the Indian subcontinent are unknown. Serial genetic analyses have revealed 2 lineages of the virus, consisting of isolates from Western Africa and the other from East Africa and Asia.11 Others suggest occurrence of mutant virus strains in the Indian subcontinent or lack of herd immunity. There is no definite histologic evidence of Chikungunya neurotropism; however, this report does highlight the clinicoradiologic findings in patients with encephalomyeloradiculitis following Chikungunya infection.
References
- Received February 20, 2008.
- Accepted after revision February 24, 2008.
- Copyright © American Society of Neuroradiology