Abstract
BACKGROUND AND PURPOSE: Hyperintensities on T2-weighted images are seen in the brains of most patients with neurofibromatosis type I (NF-1), but the origin of these unidentified bright objects (UBOs) remains obscure. In the current study, we examined the diffusion characteristics of brain tissue in children with NF-1 to test the hypothesis that a microstructural abnormality is present in NF-1.
MATERIALS AND METHODS: Diffusion tensor imaging (DTI) was performed in 50 children with NF-1 and 8 controls. Circular regions of interest were manually placed in 7 standardized locations in both hemispheres, including UBO sites. Apparent diffusion coefficients (ADC), fractional anisotropy (FA), and axial anisotropy (Am) were used to differentiate quantitatively between healthy and disordered brain matter. Differences in eigenvalues (λ1, λ2, λ3) were determined to examine parenchymal integrity.
RESULTS: We found higher ADC values for UBOs than for normal-appearing sites (P < .01) and higher ADC values for normal-appearing sites than for controls (P < .04 in 5 of 7 regions). In most regions, we found no differences in FA or Am. Eigenvalues λ2 and λ3 were higher at UBO sites than in normal-appearing sites (P < .04).
CONCLUSION: With ADC, it was possible to differentiate quantitatively between normal- and abnormal-appearing brain matter in NF-1 and also between normal-appearing brain matter in NF-1 and healthy brain matter in controls, indicating subtle pathologic damage disrupting the tissue microstructure in the NF-1 brain. Higher diffusivity for λ1, λ2, and λ3 indicates that this disturbance of microstructure is caused by accumulation of fluid or vacuolation.
Hyperintensities on T2-weighted images are seen in the brains of most patients with neurofibromatosis type I (NF-1). Although many imaging techniques have been used to assess these unidentified bright objects (UBOs), their origin remains obscure.1–4 The only pathologic study performed in NF-1 so far revealed intramyelinic vacuolar changes or spongiotic myelinopathy that correlated with the hyperintensities found on T2-weighted images.5 In addition to conventional MR imaging, several studies have used diffusion-weighted imaging (DWI) with assessment of apparent diffusion coefficients (ADCs) to gain information on UBOs that cannot be assessed by inspection of conventional images alone. On the basis of the high ADC values, a widespread myelin disorder was suggested to be present in patients with NF-1.6–9 However, ADC reflects only the magnitude of the diffusion of water molecules. Although high ADC values might suggest increased water content of the brain, with ADC alone, it is not possible to examine the microstructural integrity of the parenchyma. Diffusion tensor imaging (DTI), which measures the degree and direction of molecular diffusivity, is able to detect white matter abnormalities and characterize them in terms of white matter fiber integrity.10,11
DTI generates a diffusion tensor matrix from a series of DWIs. By matrix diagonalization, the 3 eigenvalues λ1, λ2, and λ3 can be calculated. λ1 has the largest value and reflects the diffusivity parallel to a structure; λ2 and λ3 are the middle and smallest eigenvalues, respectively; and their average represents the diffusivity perpendicular to a structure. Various anisotropy indexes (fractional anisotropy [FA], axial anisotropy [Am]) can be calculated by using the eigenvalues. They describe the ratio of the eigenvalues and are scaled from zero (isotropic) to 1 (anisotropic) and reflect the microstructure of white matter tracts. Looking at the eigenvalues themselves enables specific assessment of myelin integrity, as distinct from axonal integrity.12,13 Recently, a DTI study on adult patients with NF-1 revealed higher ADC and lower FA values. Unfortunately, changes in eigenvalues were not reported.14
The purpose of this study was to examine the diffusion characteristics of brain tissue in children with NF-1 by means of DTI and to test the hypothesis that a microstructural abnormality is present in NF-1. We tested this in 3 ways: First, by assessing ADC and indexes of anisotropic diffusion, we tried to differentiate quantitatively between normal- and abnormal-appearing brain tissue in children with NF-1. Second, we examined the normal-appearing parenchyma in NF-1 to see if it is different from parenchyma in healthy controls. Third, we looked at parenchymal integrity at UBO sites and normal-appearing sites by assessment of the eigenvalues. In addition, because T2-weighted hyperintensities in the hippocampus have been suggested to have a different pathogenetic basis from classic UBOs,15 we paid special attention to the diffusion characteristics of the hippocampal hyperintensities to see if they were different from those in other regions of interest.
Materials and Methods
Subjects
Data for this study were obtained in the context of a larger study on NF-1 and cognitive functioning. All participants were recruited from the multidisciplinary NF-1 outpatient clinic of the hospital. Inclusion criteria were the following: 8–17 years of age, NF-1 diagnosis according to the criteria of the National Institutes of Health,16 and informed consent from parents and children older than 12 years of age. Exclusion criteria were the following: segmental NF-1 (because brain involvement is not certain in these patients), pathology of the central nervous system (CNS) (other than asymptomatic gliomas), deafness, severe impaired vision, use of antiepileptics, inefficient production and comprehension of the Dutch language, and severe mental retardation (intelligence quotient <48).
One hundred twenty-six children fulfilled the age criterion. Twelve children were excluded on the basis of possible segmental NF-1 (n = 3), the use of antiepileptics (n = 3), hydrocephalus (n = 3), severe mental retardation (n = 1), and inefficient production and comprehension of the Dutch language (n = 2). The remaining 114 children were invited to participate in the larger study, of which 62 consented. The study was approved by the medical ethics committee of our institution. A total of 50 of 62 children who participated in the larger study consented to MR imaging examination (21 girls; mean age, 12.2 years; range, 8.1–15.7 years; and 29 boys, mean age, 12.3 years; range, 8.0–16.5 years).
Image Acquisition
MR anatomic imaging with DTI was performed by using a 1.5T system (EchoSpeed; GE Healthcare, Milwaukee, Wis) and a dedicated 8-channel head coil. DTI data were acquired by using a multi-repetition single-shot echo-planar sequence with a section thickness of 3 mm with no gap. The DTI images were obtained in 25 gradient directions with a sensitivity of b = 1000 s/mm2, TR = 15,000 ms, TE = 82.1 ms, 1 average, FOV of 240 × 240 mm2, and a matrix of 128 × 128 resulting in a voxel size of 1.8 × 1.8 × 3.0 mm3. Acquisition time was 5:28 minutes with a total of 53 sections to cover the entire brain.
Data Collection
All images were analyzed by visual inspection by an experienced pediatric neuroradiologist to exclude CNS tumors. Hyperintense lesions on T2-weighted, fluid-attenuated inversion recovery (FLAIR), and diffusion images (b = 0 s/mm2) were classified as UBOs. When no hyperintense lesion was present, the area was scored as a normal-appearing site.
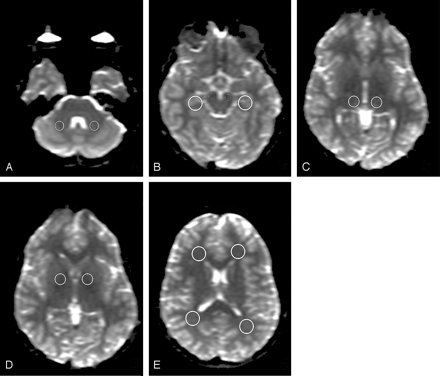
For quantitative data analysis, ADC, FA, and Am, and eigenvalues were used. FA measures the fraction of the magnitude of the diffusion tensor that can be ascribed to anisotropic diffusion.17 Am reflects the shape of the diffusion ellipsoid.18 ADC, FA, and eigenvalue maps were reconstructed by using commercially available software (Functool 3.1.23, Advanced Workstation 4.1; GE Healthcare). Circular regions of interest of specific sizes were manually placed in 7 predetermined anatomic locations in both hemispheres: the cerebral peduncle (CP), cerebellar white matter (CWM), hippocampus (HI), thalamus (TH), globus pallidus (GP), and frontal (FWM) and parieto-occipital (POWM) white matter. The size of the regions of interest was 100 mm2 for CWM; 70 mm2 for CP; 170 mm2 for HI, POWM, and FWM; and 130 mm2 for GP and TH, according to the method of Alkan et al (Fig 1).8 All region-of-interest placement was done on the b = 0 s/mm2 images because anatomic detail was better on these images than on the computed maps, ensuring anatomic precision. Regions of interest were automatically superimposed on the functional maps by the software that was used in this study. ADC maps were used to exclude CSF from measurements to minimize overestimation of the ADC values. FA maps were used when placing regions of interest in the GP and TH region to avoid as much as possible the involvement of the corticospinal tract (anterior and posterior limb of the internal capsula).
Region-of-interest placement. Transverse b = 0 s/mm2 images of a healthy control (multi-repetition single-shot echo-planar sequence; section thickness of 3 mm with no gap; 25 gradient directions; b = 1000 s/mm2; TR/TE = 15,000/82.1 ms; 1 average; FOV of 240 × 240 mm2; matrix of 128 × 128; voxel size of 1.8 × 1.8 × 3.0 mm3). Circular regions of interest are placed in the CWM (A), the CP and HI (B), the TH (C), the GP (D), and FWM and POWM (E).
Statistical Analysis
For statistical analysis ADC, FA, and eigenvalues calculated by the software were used. Am values were calculated by using the eigenvalues λ1, λ2, and λ3 in the following way: 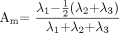
To determine intraobserver reliability of region-of-interest placement, the same observer repeated the placement in a subset of 10 scans. Single-measure intraclass correlation coefficients (ICCs) were calculated to compare the variability of data obtained.
To explore mean group differences, we averaged values obtained of the left and right hemisphere per region, per subject. All regions were then divided into the following groups on the basis of NF-1 and on the prevalence of UBOs: 1) NF-1 regions with bilateral UBOs or with a UBO on 1 site and a normal-appearing contralateral site, 2) NF-1 regions with no UBOs (both sites normal-appearing), and 3) healthy controls. Differences in ADC, FA, Am, and eigenvalues were compared by using 1-way analysis of variance or the Kruskal-Wallis test (if the distribution of the data was skewed). Significance was set at P < .05, and post hoc comparisons were done by using the Scheffé test. Differences in the proportions of λ1 and λ2, as well as the proportions of λ1 and λ3, between HI hyperintensities and UBOs in other regions, were analyzed by using the Wilcoxon signed rank test for nonparametric related samples. Statistical analysis was done by using the Statistical Package for the Social Sciences 10.0 for Windows (SPSS, Chicago, Ill).
Results
Intraobserver Reliability of Region-of-Interest Placement
Reproducibility of region-of-interest placement by a single observer proved moderate to very good, with ICCs between 0.47 and 0.91. The lowest values were found for the GP (left hemisphere, 0.47; right hemisphere, 0.70), HI (left, 0.66; right, 0.55), and TH (left, 0.52; right, 0.73), whereas the highest ICCs were found for CWM (left, 0.67; right, 0.91). However, the wide range of ICCs in these regions is disconcerting. In FWM, POWM, and CP, the ICC was >0.71.
Qualitative Evaluation of UBOs
Controls (4 girls; age range, 7.4–12.1 years; mean age, 10.8 years; and 4 boys; age range, 7.8–12.0 years; mean age, 9.6 years) were selected for comparison. All controls were without chronic disease and had normal findings on MR imaging.
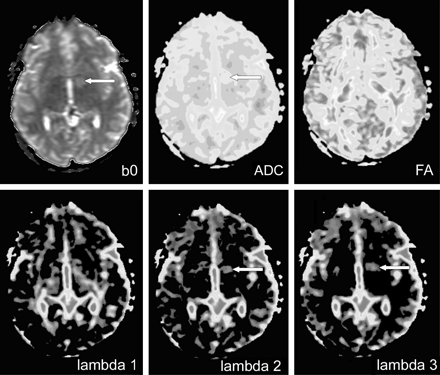
Sixty-eight percent of the children with NF-1 (n = 34) were found to have UBOs in 1 or more of the 7 selected regions, which could be detected and measured on the b = 0 s/mm2 images. Forty-six percent of the children with NF-1 had UBOs in the GP (13 bilateral, 10 unilateral), 14% in the TH (2 bilateral, 5 unilateral), 50% in the CWM (16 bilateral, 9 unilateral), and 22% in the CP (9 bilateral, 7 unilateral). The HI was visually scored bilaterally hyperintense on T2- and FLAIR images in 48% of the children with NF-1. No circumscript UBOs were found in POWM and FWM. Figure 2 presents ADC, FA, and eigenvalues maps of a girl with NF-1 with a UBO in the left GP and a normal-appearing contralateral side.
DTIs of the globus pallidus. Transverse DTIs (multi-repetition single-shot echo-planar sequence; section thickness of 3 mm with no gap; 25 gradient directions; b = 1000 s/mm2; TR/TE = 15,000/82.1 ms; 1 average; FOV of 240 × 240 mm2; matrix of 128 × 128; voxel size of 1.8 × 1.8 × 3.0 mm3). Girl with NF-1 (13 years of age) with a unilateral UBO in the GP. Arrow indicates an area of high intensity on the b = 0 s/mm2 image and high values on the ADC, λ2 (lambda 2), and λ3 (lambda 3) maps.
Measurements in the CP could not be performed in 2 children with NF-1; and in 1 of those children, motion artifacts also prohibited measurements in the HI. Mean ± SD of ADC, FA, Am, and eigenvalues per region per group are plotted in Figs 3–6.
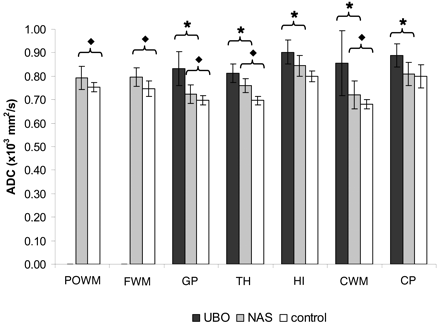
Mean ADC values. The cohort is divided in 3 groups: NF-1 regions with UBOs (UBO), NF-1 regions with normal-appearing brain matter (NAS), and controls. Mean ADC values for UBOs are higher than those for NAS in all regions (except POWM and FWM where no UBOs were found). Mean ADC values for NAS are significantly higher than those of controls in all regions, except CP. For convenience, the results of the HI area are presented in the same figure as the results of the other regions of interest. HI hyperintensities are presented as UBOs and normal-appearing HI areas as NAS. Significant differences between UBO and NAS are indicated by ★ and between NAS and controls by ⧫.
Quantitative Differentiation of Healthy and Disordered Brain Matter in NF-1
ADC values were significantly higher in regions where UBOs were present than in the matching regions with no UBOs (for all regions, P < .01). Also, ADC values were higher in NF-1 regions with no UBOs than in the regions of healthy controls, significantly so in the POWM and CWM (P < .03), FWM (P < .01), GP (P < .04), and TH (P < .01; Fig 3). To investigate the influence of region-of-interest size, we performed additional measurements in the GP region in a subset of 15 patients, by using a circular region of interest of 30 mm2. This smaller region-of-interest size resulted in a significantly higher ADC value (P < .01) as compared with the value obtained by a region of interest of 130 mm2.
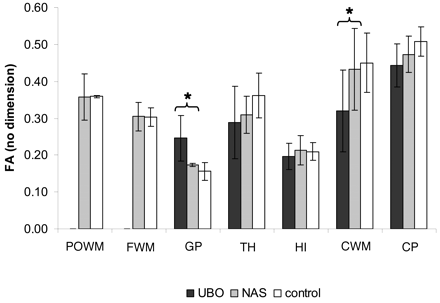
With respect to FA values, the results were not as clear-cut. Although there was a trend toward FA values in NF-1 regions, with UBOs being lower than those in matching NF-1 regions without UBOs and controls, these differences were not significant for most regions (Fig 4). Only for CWM was the FA value in NF-1 with UBOs significantly lower than that in NF-1 without UBOs (P < .01). Remarkably, results in the GP were opposite to those in other tissues, with higher FA values for NF-1 with UBOs as compared with NF-1 without UBOs (P < .01).
FA values. Mean FA values ± SD for UBO, normal-appearing sites (NAS), and controls are plotted for the 7 regions of interest. Few significant differences are found between UBO and NAS, indicated by ★.
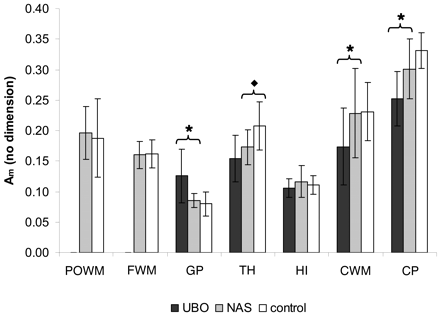
Am values were lower in NF-1 regions with UBOs as compared with NF regions without UBOs, significantly so in CWM (P < .02) and CP (P < .01). In addition, Am values were also significantly lower in NF-1 regions without UBOs as compared with controls in the TH (P < .02). In GP, an opposite trend was shown: The value found for NF-1 with UBOs was significantly higher as compared with that of NF-1 without UBOs (P < .01). No significant differences in Am were found between UBO, normal-appearing sites, and controls in the other regions assessed (Fig 5).
Am values. Mean Am values ± SD for UBO, normal-appearing sites (NAS), and controls per regions of interest. Significant differences between UBO and NAS are indicated by ★ and between NAS and controls by ⧫.
Microstructural Integrity of NF-1 Brains
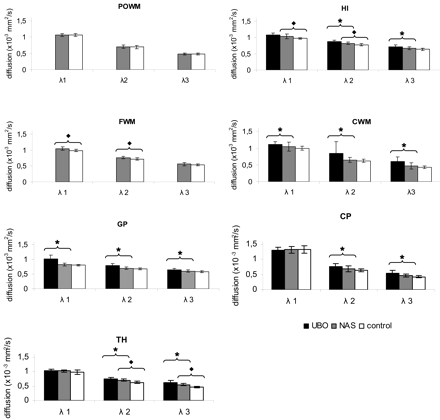
To examine microstructural integrity of the brain parenchyma in NF-1, we assessed the eigenvalues (Table). In all regions tested, NF-1 regions with UBOs had eigenvalues higher than those of NF-1 regions without UBOs (Fig 6). In CWM and GP, all 3 eigenvalues were significantly higher (P < .02); in HI, CP, and TH, λ2 and λ3 were significantly higher (P < .04), indicating a loss of microstructure of the brain parenchyma in children with NF-1 with UBOs.
Eigenvalues per region. For UBO, normal-appearing sites (NAS), and controls, mean values for the 3 eigenvalues are plotted for each region. In all regions where UBOs are found, λ1, λ2, and λ3 are higher for UBOs than for NAS and higher for NAS compared with controls. Significant differences between UBO and NAS are indicated by ★ and between NAS and controls by ⧫.
DTI values per ROI*
The eigenvalues in NF-1 regions without UBOs closely followed those in healthy controls, indicating that the microstructure is close to normal in children without UBOs, even though some slight but significant elevations were seen (FWM, P < .01 for λ1 and λ2; HI, P < .02 for λ1). The exception is in the TH, where the eigenvalues of NF-1 without UBOs were much higher than those of healthy controls, significantly so for λ2 and λ3 (P < .01).
Analysis of the difference in proportions of λ1 and λ2 for hyperintensities in the HI and for UBOs in other regions revealed that there were no differences in the proportions of λ1-λ2 for HI compared with the TH (P < .11) or GP (P < .59). The same results were found for the differences in the proportions of λ1 and λ3. This indicates that the diffusion perpendicular to the axon for HI is not different from other gray matter areas like the TH and GP. Compared with white matter areas CP and CWM, there were significantly different proportions for λ1-λ2 (P < .02 and P < .01).
Discussion
DTI in 50 children with NF-1 and 8 controls revealed significantly higher ADC values in NF-1 regions with UBOs as compared with NF-1 regions without UBOs and in NF-1 regions without UBOs as compared with controls. ADC values reflect the overall brain water content19; thus the differences between NF-1 regions with UBOs, NF-1 regions without UBOs, and controls found in this study can primarily be explained by increased water content of the brain parenchyma in NF-1, which is apparently exacerbated in NF-1 regions with UBOs. These findings confirm those of previous reports.6–9,14 However, the ADC values found in this study are not as high as the ADC values found previously. An explanation of lower ADC values may be found in the shorter effective TE and the different b-value that were used in our scanning protocol compared with reported DWI protocols.20 Also DTI improves the ability to avoid partial volume effects of CSF by fine-tuning the region of interest placement by simultaneous use of b = 0 s/mm2 images, ADC, and FA maps. Another important technical issue is the chosen size of the region of interest because it influences the ADC values. Larger regions of interest result in lower ADC values because the value represents an average of more voxels, the area of the UBO and surrounding tissue, as demonstrated in the GP by the subtest in which a region of interest of 30 mm2 instead of 130 mm2 was used.
DTI may facilitate a better understanding of the abnormalities seen in NF-1 because evaluation of microstructural integrity of the parenchyma can be achieved by assessing anisotropy indexes and eigenvalues. Anisotropy can be influenced by factors such as axon packing, relative membrane permeability to water, internal axon structure, myelination, and tissue water content.21 ADC values in this study were higher in NF-1 regions with UBOs and in NF-1 regions without UBOs than in healthy controls, suggesting increased tissue water content or decreased axon packing. However, on the basis of FA values in our study, it was only possible to differentiate between NF-1 regions with UBOs and NF-1 regions without UBOs in the CWM and GP. Remarkably, in the latter, we found higher FA values for NF-1 with UBOs as compared with NF-1 without UBOs and controls, which is a counterintuitive finding.
When carefully re-examining region-of-interest placements in the GP, we realized that it is almost impossible to avoid partial volume effects of the posterior limb of the internal capsula, even when regions of interest are drawn smaller (30 mm2 instead of 130 mm2). UBOs are typically found very near or in some cases in the internal capsula. The high anisotropy of the internal capsula affects the measured FA values for the GP. Low ICC and high variability (Fig 3) also show the difficulties of taking measurements in the GP region. In contrast to our results in children with NF-1, a recently published study on adult patients with NF-1 by using DTI found significantly lower FA values in NF-1 brains than in healthy brains, indicating generalized microstructural alterations and dysmyelination in adult patients with NF-1.14 A caveat of the study in adult patients is that it did not assess alterations of the eigenvalues, and it is, therefore, not possible to relate changes in FA to dysmyelination. Lower FA values at UBO sites might also be caused by damage to the axon as shown by MR spectroscopy.22 The study in adult patients found severely reduced concentrations of N-acetylaspartate at UBO sites, indicating that an increased myelin turnover was present, which could lead to subsequent axonal damage in adult patients with NF-1. We found no evidence for axonal damage in children with NF-1, which might be an explanation of why we did not find lowered FA values in our study.
We also did not find differences in the shape of the diffusion ellipsoid, when looking at Am values, between children with NF-1 with or without UBOs or healthy controls in most regions of interest. Although of all anisotropy indexes, Am shows the strongest trend in relative changes,23 in this study, Am was only slightly more sensitive than FA. The homogeneous attenuated white matter structures in CWM and CP in contrast to GP, TH, and HI, where the tissue also contains gray matter, which has zero anisotropy,24 could be the reason why we did find differences in anisotropy in CWM and CP between NF-1 with UBOs and NF-1 without UBOs, but not in the other regions of interest. If the eigenvalues λ1 and λ2 and/or λ3 changed in the same direction, as found in our study, no differences in FA and Am would be observed (but the change in ADC would be marked).25
Higher eigenvalues in NF-1 regions with UBOs indicate that the microstructure of the parenchyma is different from the parenchyma in NF-1 regions without UBOs. Animal studies have shown that an increment of axial diffusivity (λ2 and λ3) is related to myelin deficiency, whereas a decrease of parallel diffusivity (λ1) indicates axonal disturbance.26–28 Our findings of higher values for λ2 and λ3 indicate that diffusion perpendicular to the white matter structure is higher. Because we did not find lower values for λ1 in NF-1 regions with UBOs than in NF-1 regions without UBOs, we hypothesize that the observed changes of the brain tissue in NF-1 are not caused by damage to the axon, but relate to myelin deficiency. The higher value for all eigenvalues in our study, especially for λ1, has not previously been reported in human or animal studies. Accumulation of fluid hypothetically should increase the magnitude of all 3 eigenvalues from their normal values.29 Our study, therefore, indicates intramyelinic edema or vacuolar changes in the myelin. This confirms the study of DiPaolo et al,5 which was the only pathologic study performed in NF-1. Autopsy and histopathologic examination performed on 3 patients with NF-1 revealed intramyelinic vacuolar changes or spongiotic myelinopathy that correlated with the hyperintensities found on T2-weighted images. No stainable material was found within the vacuoles, which suggests that in life, they are filled with water.5
Notably, our study is not a radiology-pathology correlation study. To our knowledge, the specificity of changes of eigenvalues due to myelin disturbance in humans has not been tested. Because we were not able to perform histopathologic examinations in our cohort, no proof can be provided for the suggestion of myelin deficiency or vacuolar changes, however likely that eigenvalues will allow an assessment of myelin.
HI hyperintensities were of special interest in our study because a different pathogenetic basis from classic UBOs was suggested.15 Impairments in learning and behavior in mouse models of NF-1 are thought to be suggestive of disordered hippocampal functioning.15 We measured DTI parameters to examine if there is an underlying microstructural change in the hippocampal region distinct from other regions of interest. We found higher ADC values in hyperintense-appearing hippocampal areas than in normal-appearing hippocampal areas, which could explain disordered hippocampal functioning, but no differences in FA and Am. Although anisotropy indices in HI were lower compared with other regions of interest, eigenvalues showed no different pattern when comparing HI with other gray matter structures like GP and TH. Therefore, distinct pathogenesis between hyperintense HI and classic UBOs cannot be concluded in this study by using DTI parameters.
Our study has several limitations such as a limited number of children with NF-1 with and without UBOs and an even smaller number of control subjects. However, post hoc power analyses showed that power was >0.80 for all analyses in which children with NF-1 with and without UBOs were compared with healthy controls for ADC values. Although our findings contribute to the unraveling of UBOs, for we have been able to prove that the high ADC values in UBOs observed in previous publications are due to increased axial diffusivity, no histologic correlation with the observed diffusion signal-intensity abnormalities in NF-1 could be provided.
Conclusion
On the basis of the results obtained in the current study, it can be concluded that it is possible to quantitatively differentiate between healthy and disordered brain parenchyma in children with NF-1 by using ADC values. Although no differences were found in anisotropy indexes, higher values for λ2 and λ3 in NF-1 regions with UBOs than in NF-1 regions without UBOs indicate higher axial diffusivity because of less obstruction (presumably due to water accumulation in myelin). The higher λ1 contradicts axonal disturbance. The observed high diffusivity for all 3 eigenvalues (λ1, λ2, and λ3) in NF-1 regions with UBOs, as compared with NF-1 regions without UBOs and controls, supports pathologic findings and could be explained by a disturbed microstructure of the NF-1 brain due to accumulation of fluid or vacuolation when UBOs are present. A distinct pathogenesis between hyperintense HI and classic UBOs was not found in this study by using DTI parameters.
Acknowledgments
We thank all children and their parents for their participation, all technicians who performed MR imaging, and the representatives of the NF-1 clinical workgroup in the departments of pediatric dermatology, pediatric ophthalmology, general pediatrics, pediatric neurology, and clinical genetics.
Footnotes
This work was supported by grants from the Dutch association for brain research (Hersenstichting Nederland), the Sophia Children's Hospital Fund, and Erasmus MC.
Paper previously presented in part at: European Congress of Radiology, March 9–13, 2007; Vienna, Austria (7-minute oral presentation); and European Society of Pediatric Radiology, June 4–8, 2007; Barcelona, Spain (3-minute oral presentation).
References
- Received August 16, 2007.
- Accepted after revision October 24, 2007.
- Copyright © American Society of Neuroradiology