Abstract
BACKGROUND AND PURPOSE: Wingspan is a self-expanding, microcatheter-delivered microstent specifically designed for the treatment of symptomatic intracranial atherosclerotic disease. Our aim was to discuss the effect of patient age and lesion location on in-stent restenosis (ISR) rates after percutaneous transluminal angioplasty and stenting (PTAS) with the Wingspan system.
MATERIALS AND METHODS: Clinical and angiographic follow-up results were recorded for all patients from 5 participating institutions. ISR was defined as >50% stenosis within or immediately adjacent (within 5 mm) to the implanted stent and >20% absolute luminal loss. For the present analysis, patients were stratified into younger (≤55 years) and older (>55 years) age groups.
RESULTS: ISR occurred at a rate of 45.2% (14/31) in the younger group and 24.2% (15/62) in the older group (odds ratio, 2.6; 95% confidence interval, 1.03–6.5). In the younger group, ISR occurred after treatment of 13/26 (50%) anterior circulation lesions versus only 1/5 (20%) posterior circulation lesions. In the older group, ISR occurred in 9/29 (31.0%) anterior circulation lesions and 6/33 (18.2%) posterior circulation lesions. In young patients, internal carotid artery lesions (10/17 treated, 58.8%), especially those involving the supraclinoid segment (8/9, 88.9%), were very prone to ISR. When patients of all ages were considered, supraclinoid segment lesions had much higher rates of both ISR (66.6% versus 24.4%) and symptomatic ISR (40% versus 3.9%) in comparison with all other locations.
CONCLUSION: Post-Wingspan ISR is more common in younger patients. This increased risk can be accounted for by a high prevalence of anterior circulation lesions in this population, specifically those affecting the supraclinoid segment, which are much more prone to ISR and symptomatic ISR than all other lesions.
Endovascular treatment of symptomatic intracranial stenosis has recently progressed with the availability of the Wingspan stent (Boston Scientific, Natick, Mass), a self-expanding, microcatheter-delivered stent specifically designed for the cerebrovasculature.1 Although accumulated experience has documented the periprocedural safety of this treatment, the results of our recent study gave rise to concerns about durability, with relatively high rates of in-stent restenosis (ISR, 29.7%) and complete stent thrombosis (4.8%) observed at follow-up.2 In our initial analysis of patient demographics, vascular risk factors, lesion characteristics, and procedural details, we had identified anterior circulation location as an overriding factor in predicting Wingspan ISR.2
Although the average ages of patients with ISR (60 years) and without ISR (63 years)2 were not significantly different, a more in-depth evaluation of the registry data demonstrated that the incidence of ISR was greater in younger patients than in older patients. On the basis of this observation, we decided to perform a formal subset analysis investigating the influence of patient age, as well as lesion location, on ISR after percutaneous transluminal angioplasty and stent placement (PTAS) with the Gateway percutaneous transluminal angioplasty (PTA) balloon catheter (Boston Scientific, Fremont, Calif)-Wingspan stent system.
Materials and Methods
Patient and Institutional Enrollment
All patients with symptomatic intracranial stenosis undergoing attempted treatment with the Wingspan system were prospectively enrolled into a multicenter intention-to-treat registry (US Wingspan Registry) that included the Barrow Neurologic Institute, Cleveland Clinic, State University of New York at Buffalo, University of Texas Southwestern, and University of Wisconsin. The institutional review board at each institution approved the use of the Wingspan under a Humanitarian Device Exemption, as well as the collection and sharing of registry data among the participating centers.
Data Collection
Clinical and angiographic data were typically collected at the time of the initial procedure and at 3–6 months and 12–15 months thereafter, according to a standardized follow-up protocol. Clinical data were also collected at discharge and between 2 and 6 weeks after the original procedure.
Stent-Placement Technique
PTAS was performed by using the Wingspan system as described previously.1 In brief, access was typically achieved through the common femoral artery. Almost all procedures were performed through a 6F guiding catheter or long-sheath system. Heparinization was instituted to a targeted activated coagulation time of 250–300 seconds. In most cases, after conventional catheter-based angiography, an SL-10 (Boston Scientific), Prowler-10 (Cordis, Miami Lakes, Fla), or Echelon-10 (ev3, Irvine Calif) microcatheter was manipulated across the target lesion by using a 0.014-inch Synchro (Boston Scientific) or Transcend EX Soft Tip (Boston Scientific) microwire. The microcatheter was then exchanged over a 0.014-inch exchange microwire for a Gateway angioplasty balloon. The remaining lesions were primarily crossed with the Gateway angioplasty balloon and an exchange-length 0.014-inch microwire. In each case, the balloon diameter was sized to 80% of the “normal” parent vessel diameter. The balloon length was selected to match the lesion length. Angioplasty was typically performed with a slow graded inflation of the balloon to a pressure of between 6 and 12 atm for approximately 120 seconds. Following angioplasty, the balloon was removed and conventional angiography was repeated.
Next, the Wingspan delivery system was prepared and advanced over the exchange wire across the target lesion. The stent diameter was sized to exceed the diameter of the normal parent vessel by 0.5–1.0 mm. The stent length was selected to equal or exceed the length of the angioplasty balloon and to cover completely the entire diseased segment. The diameter of the stenotic lesion was measured by using biplane angiography and compared with a reference diameter of the normal vessel (usually proximal to the lesion) per the technique used in the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study.3
All patients were pretreated with antiplatelet agents (aspirin and clopidogrel); most were discharged on both aspirin (325 mg daily) and clopidogrel (75 mg daily). The dual antiplatelet regimen was usually maintained until follow-up angiography was performed. Provided that no in-stent restenosis had developed, clopidogrel was usually discontinued after follow-up angiography. All patients remained on aspirin therapy (325 mg daily) indefinitely after treatment.
Follow-Up Imaging
Most of the lesions (n = 76) were evaluated at follow-up by means of conventional catheter-based angiography. In some patients, lesions were evaluated with CT angiography (n = 21). If the entire stented segment and the proximal and distal parent vessel were well visualized on CT angiography, these stents were designated as demonstrating “no ISR.” If a region of the stented segment or adjacent parent vessel could not be well visualized, conventional angiography was performed. In patients in whom noninvasive imaging findings were ambiguous or suggested ISR, conventional angiography was performed. In 2 patients, only MR angiography was available as a follow-up examination.
If the stent construct was completely thrombosed at follow-up (n = 4 patients, 5 lesions), the lesion was excluded from the current analysis of ISR because it was not possible to confidently determine whether ISR preceded complete stent thrombosis in these cases. Follow-up imaging was available for 99 lesions. Of the 99 lesions with imaging follow-up, 93 were included in the present assessment—6 were excluded either due to complete thrombosis (n = 5) of the construct or indeterminate CT angiographic imaging (n = 1), still pending conventional angiographic follow-up for definitive adjudication.
At angiographic follow-up, the minimal luminal diameter was identified and measured. The percentage of residual or recurrent stenosis was again calculated by using the WASID technique.3 ISR was defined as a lesion demonstrating 1) >50% stenosis (ie, within, or immediately [within 5 mm] adjacent to the stent), and 2) >20% of absolute luminal loss at follow-up imaging. This second criterion was added because some lesions were left with residual stenoses measuring between 30% and 50% after the initial treatment. In these cases, a relatively small degree of luminal loss (ie, <20%) could result in ISR if a binary criterion of >50% stenosis at follow-up was used. The measurements were made by the authors at each institution and then adjudicated by 1 investigator (D.F.). Lesion retreatment was performed at the discretion of the primary operator.
For the purposes of the current study, the “supraclinoid segment” of the internal carotid artery (ICA) was used to characterize all lesions located between the origin of the ophthalmic artery and the carotid terminus. More proximal ICA lesions were characterized as either “cavernous” or “petrous” segment lesions.
Results
To date, we have treated 155 lesions in 144 patients. After stratification by age, there were 44 lesions treated in the 55 years of age and under group (average age, 47.9 years) and 111 lesions treated in the over 55 years group (average age, 68.3 years). In patients older than 55 years of age, the lesions treated were equally distributed between the anterior and posterior circulations (56 anterior, 55 posterior; 50.5% anterior circulation distribution). In patients 55 years of age or younger, the lesions were distributed predominantly within the anterior circulation (35 of 44 lesions treated, 79.5%, P < .0010).
For the 93 treated lesions with imaging follow-up meeting the criteria for inclusion in the present analysis, the average time between the procedure and the follow-up imaging study was 7.3 months, with a range of 2–18 months. Three of the 93 lesions included in the analysis were imaged at time points <3 months after stent placement (1 with ISR and 2 with no ISR). Early imaging was typically performed to assess recurrent or new neurologic symptoms.
Of the 93 lesions with follow-up, 31 (33.3%) were in patients 55 years of age or younger and 62 were in patients older than 55 years (Table). ISR developed in 14 (45.2%) stents in the younger group and in 15 (24.2%) in the older group (odds ratio [OR], 2.6; 95% confidence interval [CI], 1.0–6.5). In the younger group, ISR occurred after the treatment of 13 of 26 (50%) anterior circulation lesions versus only 1 of 5 (20%) posterior circulation lesions. Five of 14 ISR lesions were symptomatic, and all 5 were distributed within the anterior circulation. In the older group, ISR occurred in 9 of 29 (31.0%) anterior circulation lesions and 6 of 33 (18.2%) posterior circulation lesions. Four of 14 of these ISR lesions were symptomatic, with 3 of the 4 distributed within the anterior circulation.
Summary of data for 92 Wingspan stents with imaging follow-up
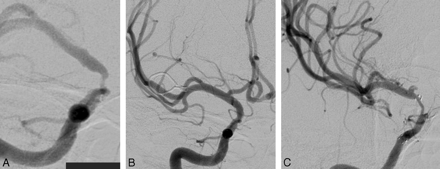
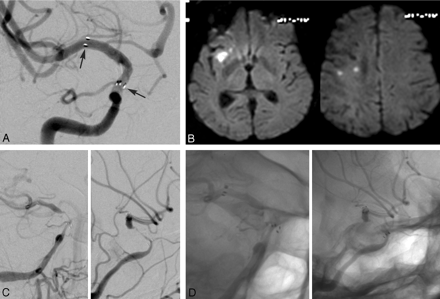
Further consideration of the anterior circulation breakdown in the younger patients demonstrated that lesions within the ICA circulation were particularly prone to ISR (10 of 17 ICA lesions treated, 58.8%). This was especially true for those lesions involving the supraclinoid segment of the ICA, with 8 of 9 (88.9%) developing ISR at imaging follow-up (Fig 1). These supraclinoid segment ISR lesions were symptomatic in 4 of 8 patients in the younger group and accounted for 80% of all symptomatic ISR lesions in younger patients (Fig 2).
A 51-year-old woman initially presented with a right middle cerebral artery distribution stroke. A, Pretreatment angiography demonstrated an irregular stenosis of the supraclinoid segment of the ICA. B, The patient underwent successful PTAS with Wingspan. C, Routine follow-up angiography at 3 months demonstrated long-segment ISR, which was successfully treated with angioplasty.
A 55-year-old woman, who initially presented with right-hemisphere watershed infarcts while taking aspirin, had additional transient ischemic attacks while taking aspirin, clopidogrel, and heparin during hospitalization. A, Initial imaging evaluation demonstrated a high-grade right supraclinoid ICA stenosis that was successfully treated with Wingspan PTAS (arrows depict the stent end markers). B, The patient returned with a history of falls and episodic left-handed and arm numbness and weakness 3.5 months after treatment. MR with diffusion imaging demonstrated several small right-hemisphere infarctions. C, Angiography demonstrated long-segment high-grade in-stent restenosis (right anterior oblique [left] and lateral [right] subtracted images). D, Corresponding right anterior oblique (left) and lateral (right) native images. This lesion was successfully retreated with angioplasty.
In the older patients, ISR developed in 4 of 15 ICA lesions, including 2 of the 6 supraclinoid ICA lesions treated. Both supraclinoid ICA lesions with ISR in the older patient group were symptomatic.
If both age groups are considered together, 10 of 15 supraclinoid ICA lesions treated went on to develop ISR, with 6 of the 10 being symptomatic. The rate at which ISR developed after the treatment of supraclinoid ICA lesions (66.6%) was far greater than the rate in all other locations (24.4%; OR, 6.2; 95% CI, 1.9–20.5). The rate of symptomatic ISR after the treatment of supraclinoid ICA lesions (6 of 15 lesions treated, 40.0%) was also considerably greater than that observed in all other distributions (3.9%; OR, 16.7; 95% CI, 3.5–78.4).
The overall rate of ISR in patients after Wingspan treatment in our series thus far (29 of 93 lesions) is 31.2%, with a rate of symptomatic ISR of 9.7% (9 of 93 lesions). If young patients with supraclinoid ICA stenosis are excluded from the analysis, the ISR rate is reduced to 25%, with a rate of symptomatic ISR of 6%. If all supraclinoid ICA stenoses are excluded from analysis, regardless of patient age, the ISR rate falls to 24.4%, with a 3.9% rate of symptomatic ISR.
Discussion
The most important findings of the present study are the following: 1) Younger (≤55 years) patients with symptomatic intracranial stenosis presenting for PTAS tend to have anterior circulation lesions, whereas older patients (>55 years) have an equal distribution of anterior and posterior circulation lesions. 2) Lesions involving the supraclinoid ICA, particularly in young patients, are extremely prone to ISR after treatment—occurring in almost 90% of the young patients treated in this series. 3) Although ISR is typically asymptomatic in other locations, ISR affecting the supraclinoid ICA is more often symptomatic (60%) than not. 4) The overall rates of ISR, and particularly the rate of symptomatic ISR, after Wingspan can be markedly reduced if supraclinoid ICA lesions are excluded from treatment.
Lesion Location and Age: Basis for Analysis
A recent evaluation of interim data from the Wingspan registry did not reveal any underlying characteristics that exposed patients to an increased risk of ISR with the exception of lesion location2—with anterior circulation lesions being much more prone to ISR than posterior circulation lesions. This initial analysis included a consideration of vascular risk factors, type of qualifying event, and procedural details.
In this dataset, the average age for patients experiencing ISR (60 years) was only 3 years younger than that for those without ISR (63 years).2 Despite the similar average ages, a more in-depth evaluation of the age distribution of patients revealed a sizeable cluster of younger patients (<55 years) with ISR. At the same time, patients without ISR were more uniformly distributed across all ages. For this reason, we sought to perform a formal subset analysis to specifically assess the influence of patient age and lesion location on ISR.
Supraclinoid Segment of the ICA Lesions in Young Patients
In the younger patient group, stenotic lesions presenting for treatment were typically located within the anterior circulation, especially the ICA, with many distributed within the supraclinoid segment (11 of 44 lesions treated in young patients). These lesions responded particularly poorly to interventional therapy, with most developing ISR and many presenting with recurrent symptoms. On the basis of these observations, we suggest that PTAS with the Wingspan system may not be an optimal treatment strategy for symptomatic stenoses affecting the supraclinoid ICA, particularly in young patients. Correspondingly, it might be advisable to exclude such lesions from any future trials of drug therapy versus PTAS with Wingspan—at least until more data are available.
Our observations raise the possibility that symptomatic intracranial stenosis affecting the supraclinoid ICA in younger patients may fundamentally differ from atherostenotic lesions distributed elsewhere and in older patients. We hypothesize that these supraclinoid ICA lesions represent more of an inflammatory arteriopathy than a primary atherosclerotic process. These lesions may fall somewhere within the spectrum of a “Moyamoya” type of arteriopathy, which characteristically occurs in younger patients, affects the supraclinoid ICA, and spares the posterior circulation.
Alternative Endovascular Treatment Options
Although Wingspan represents the only approved system for intracranial PTAS, it is possible that other endovascular treatment strategies may be better suited for those lesions that are most prone to ISR after Wingspan. The available data for angioplasty alone4–6 suggest that it is both safe and effective. Wojak et al6 observed a lower rate of “restenosis” after PTA alone for anterior circulation lesions (14.3%) in comparison with posterior circulation lesions (42.5%), though the supraclinoid segment lesions were not specifically partitioned out in this series. In addition, retreatment after PTA failure is considerably easier than that after stent placement with Wingspan.
Although no drug-eluting self-expanding intracranial stents are currently available, the existing balloon-mounted coronary drug-eluting stents have been applied with some success to treat intracranial atherosclerotic disease7,8 and may represent another reasonable option for selected lesions. At the same time, balloon-mounted coronary stents have typically been associated with high periprocedural complication rates,9 which could overcome the benefits yielded by the expected reduction of ISR. Also, recent uneasiness about the late thrombosis of these devices and recommendations for long-term dual antiplatelet therapy for patients with drug-eluting stents represent additional important concerns.10
Limitations
The primary limitations of the present study are that angiographic follow-up of all patients has not been completed at this time, and the absolute number of patients with follow-up is relatively small after they are split into subsets for analysis. At the same time, these limitations in statistical power are compensated by the very robust nature of the trends identified. Second, although based on an assessment of our initial dataset, the selection of 55 years as an age cutoff for the stratification of “young” and “old” patient groups is somewhat arbitrary. Although the observed phenomena are likely on an age continuum, a much larger dataset would be required to define more confidently the age that would best stratify patients with symptomatic intracranial stenoses in terms of their risk for supraclinoid segment ICA lesions and ultimately in-stent restenosis. Third, it could be argued that our definition of ISR (requiring both >50% stenosis and 20% late luminal loss) is too conservative and could potentially create a bias in favor of Wingspan. These criteria were instituted to avoid assigning minimal changes in absolute luminal diameter as “ISR.” This applies specifically to those cases in which the immediate post-treatment residual stenosis is greater than 35%. In these cases, insignificant, submillimeter differences in the measurement of the minimum luminal diameter, possibly related to actual minimal luminal loss, or even differences in angiographic projection or measurement error, could satisfy the definition of ISR if simple binary (>50%) criteria were applied. By applying the stated criteria, we have sought to avoid obscuring the actual rate of ISR with these marginal cases.
Conclusions
Patients 55 years of age and younger were at a higher risk for ISR after Wingspan PTAS than were older patients. The significantly higher rate of ISR associated with the treatment of supraclinoid ICA lesions accounted for this increased risk. ISR occurring in this location was also more likely to be symptomatic than that occurring elsewhere in the cerebrovasculature. On the basis of the current data, we suggest that symptomatic stenosis of the supraclinoid ICA (particularly in young patients) is not optimally treated with the Wingspan system. The rates of ISR, and particularly symptomatic ISR, after Wingspan PTAS can be substantially reduced by avoiding these lesions.
Acknowledgments
We thank Paul H. Dressel for preparation of the illustrations.
Footnotes
The US Wingspan Registry is supported by a research grant from Boston Scientific. This work was supported by the following: Aagaard-Kienitz: research grant (>$10,000)-Micrus Endovascular, Consultant/Advisory Board (<$10,000)-Micrus Endovascular, MicroVention/Terumo; Albuquerque: Consultant/Advisory Board (<$10,000)-Micrus Endovascular; Fiorella: research grant (>$10,000), honoraria (<$10,000)-Boston Scientific; Hopkins: research grants (>$10,000)-Boston Scientific, Cordis, Micrus Endovascular; honoraria (<$10,000)-Bard, Boston Scientific, Cordis, Medsn; ownership interest (>$10,000)-APW Holding Inc, Boston Scientific, Micrus Endovascular; Consultant/Advisory Board-Abbott (>$10,000), Bard (>$10,000), Boston Scientific (>$10,000), Cordis (>$10,000); Access Closure (<$10,000), marketRx (<$10,000), Micrus Endovascular (<$10,000); Levy: research grants (>$10,000)-Boston Scientific, Cordis; other research support (<$10,000)-Wingspan devices, honoraria (>$10,000)-Boston Scientific, Cordis; other (>$10,000)-Abbott Vascular (carotid stent training), ev3 (carotid stent training), Zimmer Spine (patent royalties); Masaryk: none; McDougall: honoraria (>$10,000)-Boston Scientific, Consultant/Advisory Board (<$10,000)-CardioMind, Cordis Endovascular, Gore & Co, MTI/ev3; Niemann: research grant (>$10,000)-ev3 (research on Nexus coils), Consultant/Advisory Board-Boston Scientific (>$10,000), Cordis (<$10,000), ev3 (>$10,000), Micrus Endovascular (<$10,000); Pride: employment (>$10,000)-ev3 (clinical proctor), honoraria (<$10,000)-Boston Scientific; Purdy: honoraria (<$10,000)-Cordis, other (>$10,000)-Cordis (Royalties); Rasmussen: research grants (<$10,000)-Boston Scientific, ev3; honoraria (<$10,000)-Boston Scientific, Micrus Endovascular; ownership interest (<$10,000)-Chestnut Medical; Turk: research grants (>$10,000)-Biomerix, Boston Scientific, GE Healthcare; consultant-Biomerix (<$10,000), Boston Scientific (>$10,000), GE Healthcare (<$10,000); Welch: research grant (<$10,000)-Boston Scientific; Woo: none.
Author contributions: concept: all authors; acquisition of data: all authors; drafting of the manuscript: Fiorella, Turk, Levy, Albuquerque; critical revision of the manuscript for important intellectual content: all authors; approval of the final version of the manuscript: all authors; tabulation and interpretation of data: Fiorella, Turk, Levy.
References
- Received August 8, 2007.
- Accepted after revision October 9, 2007.
- Copyright © American Society of Neuroradiology