Abstract
Background and PURPOSE: We evaluated the use of MR cisternography after intrathecal administration of gadopentetate dimeglumine to detect the presence and localization of CSF leaks in 19 patients diagnosed with spontaneous intracranial hypotension syndrome according to the criteria of International Headache Society.
MATERIALS AND METHODS: Lumbar puncture with an injection of 0.5 mL of gadopentetate dimeglumine into the subarachnoid space in the lumbar area was performed. MR images of the cervical, thoracic, and lumbar regions in axial, coronal, and sagittal planes with fat-saturated T1-weighted images were acquired.
RESULTS: We observed objective CSF leakage in 17 (89%) of 19 patients. In 14 of these 17 patients, the site of dural tear was demonstrated accurately. In 3 of these 17 patients, the contrast leakage was diffuse, and site of the leak could not be located accurately. No leakage was observed in 2 patients. No complications were detected in any of the patients during the first 24 hours after the procedure or during the 6- to 12-month follow-up.
CONCLUSION: The current results demonstrate the relative safety, accuracy, and feasibility of intrathecal gadolinium-enhanced MR cisternography to evaluate dural leaks.
The spontaneous intracranial hypotension (SIH) syndrome was originally described by the German neurologist Schaltenbrand1,2 in 1938 as hypoliquorrhea. The Headache Classification Subcommittee of the International Headache Society has proposed diagnostic criteria for SIH.3 Evidence of CSF leakage was accepted as one of the main criteria for SIH diagnosis according to International Classification of Headache Disorders.3 Although many patients with SIH recover without intervention, many do not.4,5 Some of these patients do not respond to multiple epidural blood patches and may require more targeted epidural injections, infusions, or surgical repair.4–6 In these patients, confirmation of CSF leak, localization of the actual site or sites of CSF leak, and characteristics of the dural leaks become important. Despite advances in imaging and the availability of several different and potentially useful diagnostic modalities, accurate demonstration of the site of the CSF leakage remains a challenge for radiologists and clinicians. The purpose of our study was to evaluate and report our initial experience in analyzing CSF leaks in SIH using MR imaging combined with intrathecal administration of a gadolinium-based contrast agent, that is, gadolinium-enhanced MR cisternography.
Patients and Methods
From January 2004 to January 2007, 19 consecutive patients (7 men and 12 women; aged 25–77 years; mean, 40 ± 5 years) with diagnosis of SIH according to the diagnostic criteria proposed by the Headache Classification Subcommittee of the International Headache Society3 were included in this study (Tables 1–3). All of the patients were observed to have orthostatic headache and at least 1 of the following symptoms and signs: tinnitus, hyperacusia, nausea, neck stiffness, and photophobia. The study protocol was reviewed and approved by the ethics committees of Istanbul University Cerrahpasa Medical School. Informed consent was obtained from all of the patients.
The diagnostic criteria for SIH proposed by Headache3 Classification Subcommittee of the International Headache Society
Summary of diagnostic features in 19 patients with a diagnosis of SIH
Summary of MR cisternography findings of 19 patients with diagnosis of SIH
All of the patients were first treated symptomatically with bed rest and increased fluid and caffeine intake. After 1–2 weeks of symptomatic treatment, if a patient responded poorly to this treatment, we then performed intrathecal gadolinium-enhanced MR cisternography to detect the exact location of the dural leak and its characteristics. One week after the MR cisternography, all of the patients were treated by applying an epidural blood patch. All of the patients responded within 72 hours of this application (immediate relief, not complete cure of disease). As a result, all of the patients included in this study fulfilled the SIH criteria (Tables 1) proposed by Headache Classification Subcommittee of the International Headache Society.3
All of the lumbar puncture procedures were performed under fluoroscopy. Because we had already performed a lumbar puncture, additional CSF was not withdrawn. Saline (4 mL) mixed with a single volume of gadopentetate dimeglumine (0.5 mL, Magnevist; Schering, Berlin, Germany; 469.01 mg of gadopentetate dimeglumine per milliliter) was injected into the subarachnoid space, and the needle (22-ga spinal needle) was removed. The patients stayed in elbow-knee position for 15 minutes after injection. One hour after the injection, with the patient in a supine position, fat-saturated T1-weighted images were obtained in 3 orthogonal planes (cervical, thoracic, and lumbar). Coronal and sagittal T1-weighted images (500/17 TR ms/TE ms; 2 signals acquired) and axial T1-weighted images (600/17 TR ms/TE ms; 2 signals acquired) were obtained using a 1.5T MR unit (Siemens Medical Systems, Erlangen, Germany). All of the patients were observed in the hospital for 24 hours after the procedure. The authors (S.A., H.O.) reviewed all of the images. After the patients had returned to the ward, hourly checks were made for headache progression, gross behavioral alterations, neurologic impairment, changes in mental status, subjective complaints, and vital signs, as well as for more serious events such as seizure activity and anaphylactoid reactions. Assessments were made in comparison with the baseline findings before MR cisternography. In addition, monthly clinical neurologic follow-up was performed for 12 months. Follow-up was undertaken by the neuroradiologist (S.A., F.K., S.B., and H.O.) in charge of the project.
Results
No patients showed gross behavioral changes, neurologic impairment, alterations in mental status; changes in vital signs from baseline, anaphylactic, or other allergic reaction; or seizure activity at any time during the initial 24 hours after intrathecal gadolinium-enhanced MR cisternography or at monthly follow-up for 12 months. Increase in orthostatic headache seen in 5 patients after the procedure is related to lumbar puncture performed, and these new symptoms resolved spontaneously after 36 hours of lying flat.
In all of the patients, the gadopentetate dimeglumine entered the subarachnoid space at the spinal level and enhanced the subarachnoid space totally. Erroneous, pure, epidural injections were not performed. We observed objective CSF leakage in 17 (89%) of 19 patients (Table 3). In 14 of these 17 patients with CSF leakage, the site of dural tear was accurately demonstrated (Fig 1). However, in the remaining 3 patients, as the result of a high-volume fistula, diffuse epidural and paravertebral accumulation of the contrast medium impeded our locating the exact site of dural tear (Table 3).
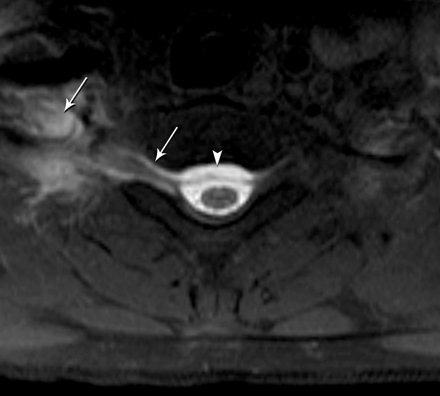
Axial T1-weighted fat-saturated MR image shows a right-sided CSF leak at the cervicothoracic junction extending into the right paraspinal soft tissue (arrows) and epidural collection (arrowhead).
In patients in whom the tear could be accurately localized, 10 demonstrated a single tear site, whereas the remaining 4 patients demonstrated multiple tear sites. In patients with a single tear site, the level of the tear was thoracic in 7 patients, lumbar in 2 patients, and cervical in 1 patient. There were 2 tears in 3 patients and more than 2 tears in 1 patient. In patients with diffuse leakage of CSF, 2 had dorsolumbar and 1 had dorsal involvement (Fig 2).
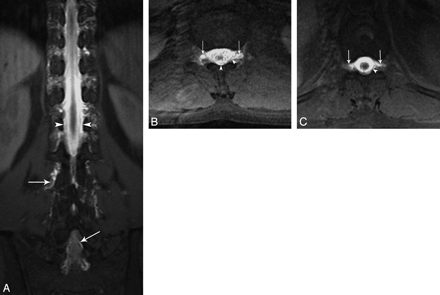
Coronal (A) and axial (B and C) T1-weighted fat-saturated MR images reveal diffuse epidural (arrowhead) and paravertebral contrast accumulation (arrows) at the thoracolumbar area.
Of 17 patients, 13 demonstrated concomitant epidural and paravertebral contrast leakage. In the other 4 patients, the leakage site was paravertebral without prominent epidural involvement. One or more meningeal diverticula at the spinal level were demonstrated in 11 patients. A cervical disk and dural tear at this level were detected in 1 patient, and 1 patient was detected as having a thoracic dorsal disk.
Discussion
In patients unresponsive to conservative approaches or to an initial epidural blood patch, it is essential to demonstrate not only leakage but also the site and morphology. This is because the treatment strategy differs dependent on the location of the leak or leaks (dorsolumbar versus cervicothoracic) and the morphology (blood patch versus surgery). Although CSF leakage can be detected intraoperatively, exact localization of the dural tear may not be possible in some cases.6,7 In such a case, the surgeon must be satisfied simply with packing the epidural region. This reflects on the importance of detecting dural tears preoperatively, which may not be demonstrated intraoperatively. Before the introduction of imaging modalities, SIH syndrome was thought to be the result of decreased CSF production or hyperabsorption. However, the underlying cause of SIH is now understood as being caused by a CSF leak.4–6 Detection of dural leaks is important both in diagnosing SIH and in determining the best treatment strategy. Evidence of CSF leakage was accepted as one of the main criteria for diagnosis of SIH according to the International Classification of Headache Disorders.3
Evidence of CSF leakage is currently detected by conventional myelography, radionuclide cisternography (RC), or CT cisternography. Conventional myelography is now obsolete, because it lacks cross-sectional features and anatomic superposition. RC is an indirect and direct confirmatory test of SIH. The presence of SIH is indirectly proven by the lack of injected dye ascending, rapid disappearance of the radioisotope from the CSF, and an early appearance of the radioisotope in the urinary bladder.8,9 However, the leakage site may not be demonstrated in lumbar cisternography, and several reports have shown the inability of RC in demonstrating the site of leaks.6,8,9 Other disadvantages of RC are its poor spatial resolution, its invasiveness, and the possible radioisotope extravasation through the needle tract that can result in inaccuracy in its interpretation.10
CT cisternography is considered the most reliable imaging technique for localizing the actual site or spinal level of a CSF leak.5–7 In a study by Mokri,5 CT cisternography identified the spinal level of a CSF leak in 67% of patients compared with 50% and 55% with spinal MR imaging and RC, respectively. Because of a lack of a “gold standard” imaging study, the sensitivity or specificity of each imaging study cannot be evaluated. Spinal MR imaging frequently shows extra-arachnoid or extradural fluid collections, enhancement of cervical dura, and dilation or engorgement of the epidural venous plexus. The location of the extra-arachnoid or extradural fluid collections rarely reflects the actual site of a CSF leak.5,6,11
The BEIR VII report, published in 2005 by the largest scientific organization in the world, the National Academy of Sciences, has described a 1-in-1000 chance of developing cancer from a single radiation exposure of 10 mSv.12 Ten mSv is approximately equal to 1 CT study of the chest region, the abdominal region, or the pelvic region. The risk in children is even higher, with a reported chance of 1 in 550 of developing cancer. The judicious use of CT in children cannot be overemphasized. Judicious use of CT in adults up to the age of 40 years should be exercised. Pelvic and chest CT studies should be held to a minimum in women because of the risk to the gonads and breast tissue, respectively.12 It is obvious that the dose in CT cisternography is higher than 10 mSv, because cervical, thoracic, and lumbar regions are scanned during CT cisternography. Additional delayed scans are frequently required in a slow-flow fistula, and CT fluoroscopy is required to construct cine images for a high-volume fistula. These studies of high-dose imaging are unacceptable in SIH patients, mostly for middle-aged women. Considering the high doses and its lack of “gold-standard” sensitivity, it is evident that more reliable and safer techniques are required to detect CSF leakage.
Since the introduction of gadolinium-diethylene-triaminepentaacetic acid (Gd-DTPA) as a contrast agent for intravenous application, it has established itself as a safe means of contrast enhancement in MR imaging.13,14 Although adverse reactions from Gd-DTPA are rare, they do include nausea, vomiting, headache, anaphylactoid reactions, and seizure.15,16 In the past few years, solute Gd-DTPA also has been used to contrast cavities such as joints17 and the subarachnoid space.18–26 Intrathecal gadolinium (gadopentetate dimeglumine) is currently used to evaluate CSF fistula mostly for rhinorrhea. Jinkins et al23 first described this technique. Later, Reiche et al24 and Aydin et al25 used the same technique to evaluate CSF fistulas. In addition, a multicenter human study by Tali et al26 evaluated the safety and clinical response to gadopentetate dimeglumine in 95 patients who presented clinically with a variety of cranial or spinal signs and symptoms. These studies show the relative safety and tolerance of low-dose (0.5 mL) intrathecal gadolinium administration.23–26 Regarding the intrathecal use of other gadolinium preparations, there has been no report about their safety.
We objectively detected dural leakage, reflecting passage of contrast medium from the subarachnoid space to the epidural and/or paravertebral space in 17 patients. We could not detect any leak in 2 patients. Thus, the sensitivity of MR cisternography is approximately 17 (89%) of 19 in patients with SIH. Although no conclusive data have been reported, this rate is better than CT, which approaches 67% at best, RC (55%), and MR imaging (50%).4–6
In 10 patients, we detected a single dural tear, though in 4 patients, there were multiple dural tears. In 3 patients, contrast leakage expanding to the entire thoracic and throcolumbar areas was seen. This is probably reflective of a high-volume fistula, and, therefore, the real site of dural tear could not be detected. Contrast leakage was seen in the throcolumbar area in 2 of these patients and in the dorsal area in 1. In patients with a single dural tear, the site of tear was dorsal in 7, cervical in 1, and lumbar in 2 cases. In patients with multiple dural tears, leakage in both cervical and thoracic areas was seen in 2 patients, in the thoracic area in 1 patient, and in both the thoracic and lumbar regions in 1 patient. In the literature, leakage is usually reported as being seen in the cervical-thoracic and thoracic areas. In our study, the dorsal area generally was the site of leakage. In patients with diffuse leakage, the throcolumbar area was primarily involved.
The true incidence of diffuse leakage, which is partially discussed by Luetmer and Mokri27 in their CT cisternography study, is not known. Because of the high viscosity of CT contrast agent, it is thought that diffuse leakage of contrast agent would result in a lower detection rate in CT cisternography than that of MR cisternography. Comparative studies are required to elucidate true rates. With MR cisternography, early images (15 minutes) may partially prevent this problem. If intrathecal injection is provided in MR gantry, this will again prevent this problem.
Multiple dural tears required attention in 4 patients. In the past, the dural tear concept was thought to be a single-site regularly bordered area. However, studies have shown that these tears may be complex and multiple, despite isolated ones like lumbar puncture dural tears.6,7,28 In a recent publication by Cohen-Gadol et al7 examining 13 patients, single dural tears were seen in 8 patients. There were 2 dural tears in 3 patients, and more than 2 dural tears in 2 patients. In our study, 3 patients presented 2 dural tears, and in 1 patient, there were more than 2 dural tears. These results seem to parallel previous series.
In 13 of 17 patients in this study, epidural contrast leakage was detected. In the remaining 4, contrast leakage was seen in the paravertebral area or neural foramina without prominent epidural leakage. These findings demonstrate that tears may be proximal along the dura, as well as distal, along the radicular dural sheath.
An important point regarding this technique is that the epidural and paravertebral contrast veins become hyperintense with contrast passage. In inexperienced hands, this finding may be interpreted as a dural tear or as paravertebral contrast leakage. This finding has its own characteristics, for example, a continuous tubular shape and an anatomic tract of vein passage (medial to lateral and posterior to anterior). In addition, the needle tract, which is generally not seen on CT, and epidural contrast accumulation beside the needle tract are important landmarks for false positivity.
Actually, meningeal diverticula are thought to be involved in the etiology to dural tears. Cohen-Gadol et al7 reported meningeal diverticula in 9 (69%) of 13 patients in their series. We detected meningeal diverticula in 11 (58%) of 19 patients in this study. This rate seems to be much higher than that of CT, RC, and spinal MR imaging. MR cisternography is a more useful technique for this purpose. We detected multiple dorsal meningeal diverticula in one patient and cervical, dorsal, and lumbar giant meningeal diverticula in another patient.
This study did not compare CT, RC, spinal MR imaging, or intrathecal gadolinium-injected MR cisternography. However, we believe that the findings of 2 patients may provide a perspective. In 1 patient, RC, CT cisternography, and MR imaging all revealed multiple meningeal diverticula, some of which were giant; however, we could not detect any objective sign of leakage (Fig 3). This condition may have resulted from 2 reasons: either the leakage pattern is intermittent or there is fistula with very little output that could not be detected using MR cisternography. In another patient, CT cisternography revealed only meningeal diverticulars in the lower dorsal area with no objective sign of leakage. However, MR cisternography detected a meningeal diverticula, as well as an objective sign of leakage on right side of the L1 vertebra (Fig 4).
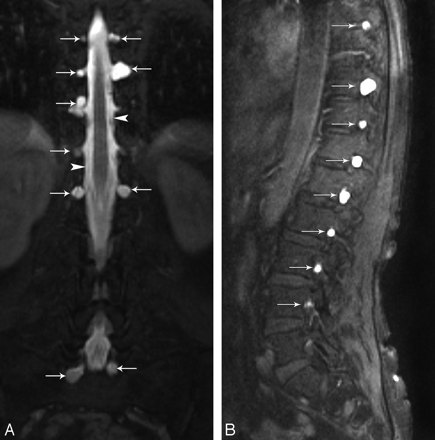
Coronal and sagittal T1-weighted fat-saturated MR images show multiple bilateral meningeal diverticula (arrows) at the thoracic and lumbar level.
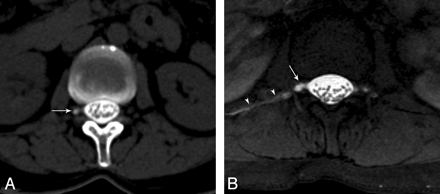
A, Axial CT cisternography image shows right meningeal diverticulum (arrow) without CSF leak at the level of L1. B, Axial T1-weighted fat-saturated MR image reveals both a CSF leak on the right side (arrowheads) and a right meningeal diverticulum (arrow).
We did not compare CT cisternography with RC because we considered it unethical for patients to undergo a second intrathecal contrast-enhanced cisternographic study solely for the sake of data collection and comparison. Intrathecal administration of a gadolinium-based contrast agent is not currently approved worldwide. Furthermore, our study represents an investigation of the use of only 1 gadolinium preparation, gadopentetate dimeglumine. Because other gadolinium-based contrast agents exist, our findings cannot be generalized to the routine intrathecal use of all gadolinium-based contrast agents. Although a relatively low-dose Gd (0.5 mL) is used in MR cisternography, it must be kept in mind that Gd can cause nephrogenic systemic fibrosis, especially in patients with chronic renal failure.
This technique does not need to be applied to all patients with classic SIH symptoms, signs, and imaging findings. In patients who do not respond to 1 or 2 epidural blood patches, this technique seems to be effective to detect and localize accurate sites of dural leak. It provides multiplanar capabilities without risk of radiation exposure. It seems to be an excellent alternative to CT cisternography. Gd-DTPA-enhanced MR cisternography offers an excellent approach to depict the anatomy of CSF spaces and CSF fistulas. However, to determine its diagnostic significance, further clinical studies evaluating greater patient numbers are necessary. In addition, long-term patient follow-up is needed to detect delayed complications, such as arachnoiditis.
References
- Received March 5, 2007.
- Accepted after revision May 24, 2007.
- Copyright © American Society of Neuroradiology