Abstract
BACKGROUND AND PURPOSE: Complications from lumbar puncture (LP) include headache; mild puncture-site pain; and, rarely, subdural, epidural, or subarachnoid hemorrhage. In infants, asymptomatic leakage of CSF documented with ultrasound is common. We report the MR imaging findings and clinical course of 25 symptomatic patients with spinal epidural collections after LP.
MATERIALS AND METHODS: MR imaging and clinical records of 25 children with new symptoms following LP were retrospectively reviewed.
RESULTS: All patients had abnormal dorsal spinal epidural collections. Signal-intensity characteristics of the collections were most commonly isointense to CSF on all pulse sequences. Significant anterior displacement of the dura with effacement of the subarachnoid space was frequently noted. All patients had fluid surrounding small foci of epidural fat, elevating them from their native interspinous fossa, resulting in a “floating” appearance. Eighteen collections involved the thoracic and lumbar spine; 4 involved the thoracic, lumbar, and sacral spine; 2 extended from the lumbar to the cervical level; and 1 was isolated to the lumbar spine. Five patients had follow-up MR imaging showing complete resolution of collections. The size of the collections was not directly related to the number of puncture attempts. Clinical symptoms resolved with time in all patients with conservative management.
CONCLUSION: Symptomatic epidural fluid collections after LP are often extensive and may compromise the thecal sac. These collections are not usually the result of a difficult LP and have signal intensity characteristics most consistent with CSF leak rather than hemorrhage. Signs and symptoms typically resolve with time, without treatment and with no serious sequelae.
Lumbar puncture (LP) is a routine clinical procedure used to evaluate patients with suspected meningitis, demyelinating diseases, and metabolic disorders. Common complications of LP include headache and mild pain at the puncture site. Uncommon complications of LP, such as epidural, subdural, and subarachnoid hemorrhage, in the absence of a known bleeding disorder, have been well documented in adults.1–5 Similar complications, including leakage of CSF, have rarely been reported in children.6–8
Our purpose was to report the clinical and MR imaging findings in 25 symptomatic children with post-LP epidural fluid collections (EDC). Kiechl-Kohlendorfer et al6 recently reported the sonographic findings in asymptomatic neonates with post-LP CSF leakage. To our knowledge, the MR imaging findings and clinical correlation in such a large population of children with symptomatic post-LP EDC have not previously been reported.
Methods
MR imaging and clinical records of 25 patients (17 male and 8 female) with post-LP EDC were retrospectively reviewed. Study subjects were identified by using the medical-notes search engine at our institution. Study subjects were gathered during a period of 8.5 years, January 1995 through June 2003. Institutional review board approval was granted for retrospective review of clinical records, images, and reports.
MR imaging was performed with a 1.5T MR imaging unit (GE Healthcare, Milwaukee, Wis). Images obtained included sagittal T1-weighted MR images (TR/TE, 400–638/9–15), sagittal fast spin-echo MR images (TR/TE, 3000–5000/108–133), and axial T1-weighted MR images (TR/TE, 550–800/9–18). Twelve of the 25 patients were imaged without contrast, 5 were imaged with contrast, and 8 were imaged both with and without contrast. The extent of the EDC was noted in terms of vertebral levels. Five patients had follow-up MR imaging in which prior EDC were evaluated as present or resolved.
The average age of the patients was 5 years 1 month (range, 5 months to 13 years) at the time of imaging. Only 1 patient had a known bleeding dyscrasia (hemophilia A), none were being treated with anticoagulation medication, and none had history of prior accidental trauma. Clinical indications for LP in these patients included the following: evaluation of fever, headache, vomiting, ataxia, seizures, diplopia, change in mental status, loss of consciousness, and lower extremity paresthesias. One patient had LP for a baclofen trial in preparation for intrathecal baclofen pump placement, and 1 patient had LP for a cisternogram to rule out an arachnoid cyst.
Patients presented post-LP with new symptoms that prompted MR imaging. Symptoms included lower back pain, leg pain, lower extremity weakness, decreased lower extremity reflexes, inability to stand, difficulty walking, unsteady gait, ataxia, paraspinal muscle spasms, paresthesia, and flexed neck position. Standard MR imaging of the spine was performed in all patients 1–7 days after LP (average, 2.3 days); 24 patients were imaged within 4 days of LP.
Results
All 25 patients had abnormal spinal EDC evident on MR imaging. All collections were located in the dorsal aspect of the spinal canal, posterior to the spinal cord and its surrounding thecal sac, and all significantly displaced the dura anteriorly. All patients had normal intrinsic cord signal intensity. Relative to CSF, all collections were hypointense on noncontrast T1-weighted images and hyperintense on T2-weighted images. The EDC did not appear to have the usual signal-intensity characteristics of acute/subacute blood but were hypothesized to be the result of a CSF leak, possibly mixed with small amounts of blood. Hypointense T1/hyperintense T2 signal intensity filled the expanded epidural space and surrounded the posterior epidural fat pads, lifting them out of their usual position between the spinous processes. This resulted in an appearance of floating fat pads. Hypointense T1/hyperintense T2 fluid also infiltrated the elevated fat pads, in some cases causing the collections to appear heterogeneous. All collections resulted in decrease or total effacement of normal CSF signal intensity surrounding the cord and/or cauda equina nerve roots (Fig 1). None of the collections caused cord compression.
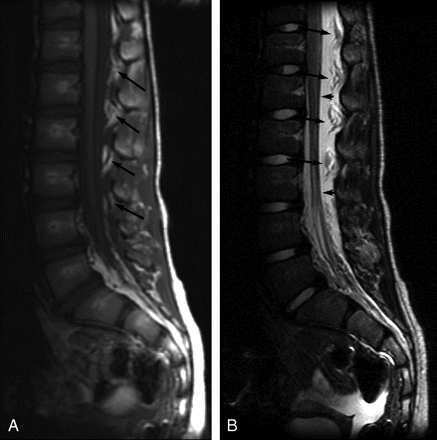
Sagittal MR images of patient 8 showing thoracolumbar EDC 1 day post-LP. A, Noncontrast sagittal T1-weighted image (TR/TE, 638.3/14) shows low-signal-intensity EDC extending from at least T11-S1. The epidural fat pads are heterogeneous (arrows) secondary to infiltrating fluid. B, Sagittal T2-weighted image (TR/TE, 3260.9/125) shows high-signal-intensity EDC elevating and infiltrating “floating” epidural fat pads (long arrows) and deviating the dura anteriorly (short arrows).
Eight patients had T1-weighted pre- and postcontrast images; 3 showed mild postcontrast dural enhancement. Five patients had only postcontrast T1-weighted images without precontrast T1-weighted images. Of these 5 patients, 3 had high signal intensity within the collections. Because none of the other collections were hyperintense on T1-weighted noncontrast images, high signal intensity may represent leakage of contrast rather than hemorrhage or contrast enhancement of abnormal fluid (Fig 2).
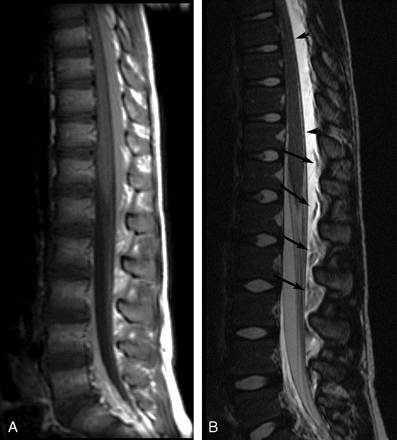
Sagittal postcontrast MR images of patient 19 showing very extensive EDC 1 day post-LP. A, Contrast-enhanced T1-weighted image (TR/TE, 500/14) shows high-signal-intensity EDC, which extended from C7-L4 (cervical images not included). B, Contrast-enhanced T2-weighted image (TR/TE, 4000/115) shows very-high-signal-intensity EDC, hyperintense to CSF, with compression of the thecal sac and anterior deviation of the dura (short arrows). Heterogeneous floating fat pads (long arrows) are more obvious on the T2-weighted image.
Twenty-four collections extended beyond the lumbar level (Fig 3). Eleven (44%) involved thoracic and lumbar levels; 4 (16%) involved the thoracic, lumbar, and sacral levels; 2 (8%) extended from the lumbar spine to the cervical spine; and 7 collections (28%) involved the lumbar and at least the lower thoracic level, with the superior-most extent not included in the images. Only 1 collection (4%) was isolated to the lumbar spine. A summary of history and imaging findings is included in the Table.
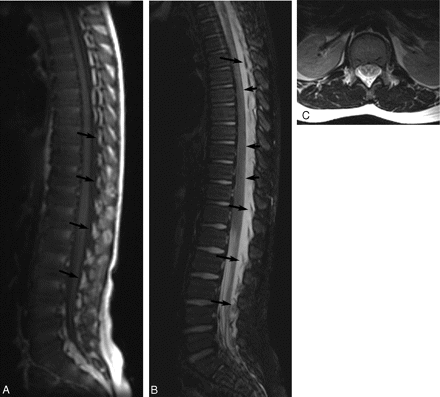
MR images of patient 2 showing extensive EDC 1 day post-LP. A, Noncontrast T1-weighted image (TR/TE, 500/9) of the spine shows a low-signal-intensity EDC extending from the upper thoracic level of the spine to the sacral level. Elevated epidural fat pads (arrows) can be seen and appear heterogeneous due to fluid infiltration. B, Noncontrast T2-weighted image (TR/TE, 4000/126) of the spine shows high-signal-intensity EDC. Epidural fat pads (long arrows) are elevated, and the dura is deviated anteriorly (short arrows). C, Axial T2-weighted image (TR/TE, 4000/126) at the level of the conus shows heterogeneous signal intensity in the dorsal epidural space secondary to fluid elevating and infiltrating the epidural fat pads. There is resultant deviation of the dura anteriorly.
Summary: history and imaging findings
The extent of the collections did not seem to be directly related to the number of puncture attempts: 1 patient who had multiple LP attempts had only collections at the thoracic/lumbar level. Several of the patients who had only 1 puncture attempt had collections extending from the cervical to the lumbar spine or from the thoracic to the sacral spine.
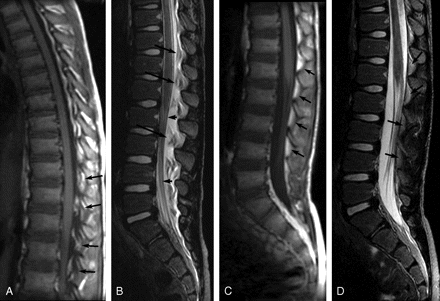
Five patients had follow-up MR imaging after the initial MR imaging discovery of EDC. The average time between initial and follow-up imaging was 3 months (range, 13 days to 6.5 months). Follow-up MR images showed complete resolution of the EDC in all 5 patients (Fig 4).
Sagittal MR images of patient 5 showing initial EDC 4 days post-LP and resolution of EDC 13 days later. A, Noncontrast sagittal T1-weighted image (TR/TE, 500/12) shows hypointense EDC extending from T12-S2. Epidural fat pads (arrows) are shown to be elevated and infiltrated with low-intensity fluid. B, Noncontrast sagittal T2-weighted image (TR/TE, 5000/135) obtained at the same time as A shows hyperintense fluid deviating the dura anteriorly (short arrows) effacing the CSF and elevating the fat pads (long arrows), which are infiltrated with fluid. C and D, Sagittal T1-weighted (C) (TR/TE, 500/12) and sagittal T2-weighted (D) (TR/TE, 5000/133) images obtained 17 days post-LP show resolution of EDC. Epidural fat pads (arrows) are normal in position and homogeneous. The dura is no longer deviated, and the intrathecal CSF is not effaced.
Clinical symptoms that led to imaging resolved in all patients with time and conservative treatment, usually analgesics and muscle relaxants.
Discussion
MR imaging of epidural and subdural spinal hematoma has been well documented in adults in the past, mostly spontaneous with associated disk disease or postepidural anesthesia. Nineteen reported cases of MR imaging of spontaneous epidural hematoma have been reported.2,9 Gundry et al2 described imaging findings and clinical correlation of 18 cases of epidural hematoma in adults: Most of these hematomas were associated with small disk herniations or annular tears, and all hematomas occupied the ventral epidural aspect of the spinal canal. On proton-attenuation and T2 MR imaging in 13 of these patients, the epidural hematomas were intermediate-to-high signal intensity. Signal intensity on T1-weighted images was not described. MR imaging findings were confirmed by surgical evacuation of the hematomas.2 Heye9 reported a case of spontaneous epidural hematoma with MR imaging in a patient taking aspirin prophylactically. Peltola et al5 reported 1 case of MR imaging of epidural hematoma post-LP in an adult. Kulkarni et al3 reviewed MR imaging of 15 cases of spinal subdural hematoma and presented 1 new case that showed an isointense T1-weighted and a hyperintense T2-weighted posterior subdural blood mass possibly mixed with CSF. Twelve patients were treated with surgical decompression, but 4 were treated conservatively, with spontaneous resolution.
Seventeen cases of spinal hematoma have been reported in children: 11 cases were post-LP and 6 were spontaneous. One child with no known coagulopathy developed severe back pain and pain on flexion of his legs after LP. MR imaging showed spinal epidural hematoma.1 He was treated with dexamathasone, clinical symptoms resolved, and follow-up MR imaging showed resolution of hematoma. Ten other cases were reported after LP in children with bleeding dyscrasia or who were on anticoagulant therapy. Three of these 10 patients were treated with laminectomy to remove the hematoma, 6 hematomas were confirmed by laminectomy at autopsy, and 1 patient had no surgical treatment and mild sequelae.1,10,11 Last, 6 spontaneous epidural hematomas have been reported in children with no known bleeding dyscrasias; these patients were also treated with laminectomy.7
We report 25 children with post-LP spinal EDC who did not have typical imaging characteristics of hemorrhage and whose EDC was thought to be primarily related to CSF leakage. We found 2 publications of sonography findings in neonates with similar findings. Kiechl-Kohlendorfer et al6 reported 21 neonates who had post-LP sonography, which indicated the presence of epidural CSF collections. None of the 21 patients in this report were noted to have clinical symptoms that led to imaging, and no MR imaging was reported. Many of these collections were extensive and markedly compressed the subarachnoid space.6 Coley et al8 reported the sonographic findings in 32 neonates and infants after failed LP attempts. Twenty-three patients had complete obliteration of the CSF space secondary to intrathecal and/or epidural echogenic material, which the authors presumed to be hematoma. Yousry et al12 reported cervical MR imaging findings in 20 adult patients with postural headache and found what they described as subdural or epidural spinal hygromas in 70% (14/20). The authors thought that the exact location of collections (subdural or epidural) was difficult to determine but that they were subdural, and they speculated that they were the result of transudate from the venous plexus rather than frank extravasation.12 We agree that the exact location of spinal fluid collections as subdural or epidural can be difficult. However, because the fluid elevated and infiltrated the epidural fat pads in our patients, we believe most of the fluid was in the epidural space.
All patients in our study presented 1–7 days post-LP complaining of 1 or more of the following symptoms prompting the clinician to order imaging: lower back pain, leg pain, lower extremity weakness, inability to stand, difficulty walking, unsteady gait and ataxia, paraspinal muscle spasms, paresthesia, and flexed neck position. EDC were identified in the lumbar spine, with almost all extending to the thoracic level and 2 extending to the cervical level. The extent of the collection did not seem to be related to the number of puncture attempts. Several of the patients had only a single puncture attempt but had larger collections that spanned the cervical, thoracic, and lumbar spine. Three of the patients, however, had multiple puncture attempts but only had collections extending from the thoracic to the lumbar spine.
Only 1 child had a known bleeding disorder (hemophilia A); this child was treated with factor VIII. No patients were receiving anticoagulants, and no patients had history of prior accidental trauma. All patients were treated conservatively (primarily with analgesics and muscle relaxants), and all had resolution of clinical symptoms that initially led to MR imaging. Five patients had follow-up MR imaging. All 5 EDC had resolved completely (within 13 days to 6.5 months later) without surgical intervention.
Conclusion
LP complications including headache and pain at the puncture site are common and resolve without the need for MR imaging. Rarely, children present with new symptoms post-LP, including lower back pain, leg pain, lower extremity weakness, decreased lower extremity reflexes, inability to stand, difficulty walking, unsteady gait, ataxia, paraspinal muscle spasms, paresthesia, and flexed neck position, which prompt MR imaging. These children frequently have symptomatic EDC that primarily appear to be secondary to CSF leakage. These collections are dorsal, often extensive, and may compromise the thecal sac. The collections have a characteristic appearance on MR imaging and appear to be directly related to transient clinical symptoms, which resolve with conservative management, with no serious sequelae.
References
- Received August 9, 2006.
- Accepted after revision March 4, 2007.
- Copyright © American Society of Neuroradiology