Abstract
SUMMARY: Lymphomatoid granulomatosis (LA) is a rare angiocentric lymphoreticular proliferative disease that primarily involves the lungs but may also involve extrapulmonary sites including the central nervous system, skin, and kidneys. It is rare for this condition to affect children, and presentation as a cerebellar mass is unusual. In this report, we describe a 10-year-old girl with biopsy-proved cerebellar LA.
Lymphomatoid granulomatosis (LG) is a rare Epstein-Barr virus (EBV)–associated B-cell lymphoproliferative process with similarities to posttransplantation lymphoproliferative disorder.1 LG is characterized by an angiocentric and occasionally angiodestructive polymorphic cellular infiltrate.2 The disease primarily involves the lungs but also may involve the skin, kidneys, and brain. Central nervous system (CNS) involvement occurs in approximately 30% of affected patients, and patients may present with nonspecific neurologic symptoms such as seizures and incontinence.3 LG affects men more commonly than women (2:1) and presents mainly in adults between the fourth and sixth decades of life, though patients of all ages may be affected. Its occurrence in children is quite rare.1,4–6 CNS involvement is typically hemispheric and may be tumorous (masses) or nontumorous (nonspecific T2 hyperintensity).4 In this report, we describe a 10-year-old girl presenting with a cerebellar mass, a most unusual location, pathologically proved to be LG.
Case Report
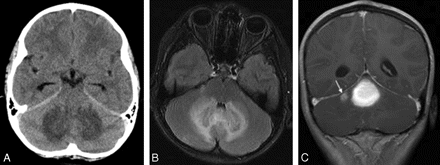
A 10-year-old girl presented with several days of frontal headache and repeated episodes of vomiting, malaise, and subsequent mild photophobia. A noncontrast head CT examination raised the possibility of a cerebellar mass (Fig 1A). When the patient arrived at our institution, CSF examination showed 63 white blood cells (90% lymphocytes) and 1 red blood cell. Subsequent cranial MR imaging demonstrated a 3.0 × 2.0 × 2.5 cm poorly marginated mass located in the anterosuperior aspect of the cerebellar vermis with extension into the medial aspects of both cerebellar hemispheres. The mass appeared hypointense on T1-weighted images and hyperintense on T2-weighted and fluid-attenuated inversion recovery images (Fig 1B) and demonstrated contrast enhancement that was greater peripherally than centrally. There was mild surrounding edema, resulting in mild compression of the interpeduncular and prepontine cisterns. A second smaller contrast-enhancing lesion was identified in the superior aspect of the right cerebellar hemisphere, measuring 1.6 × 1.0 × 0.8 cm (Fig 1C). Pediatric infratentorial malignancies were considered in the differential diagnosis, including medulloblastoma/primitive neuroectodermal tumor.
A, Axial CT scan demonstrates slight hyperattenuation of the cerebellar vermis with surrounding symmetric low attenuation involving the medial aspects of both cerebellar hemispheres. B, Axial FLAIR image shows symmetric patchy T2 hyperintense areas involving the medial aspects of both cerebellar hemispheres and the intervening cerebellar vermis. C, Postcontrast coronal T1-weighted image demonstrates a 3.0 × 2.0 × 2.5 cm mass located in the anterosuperior aspect of the cerebellar vermis with extension into the medial aspects of both cerebellar hemispheres. There is another smaller contrast-enhancing lesion in the superior aspect of the right cerebellar hemisphere, measuring 1.6 × 1.0 × 0.8 cm (arrow).
A craniotomy was performed with excisional biopsy of the midline cerebellar mass. Microscopic examination revealed atypical lymphohistiocytic proliferation. An intense infiltrate of CD3-positive T-lymphocytes, CD68-positive histiocytes and microglia, polyclonal plasma cells, relatively fewer small CD20-positive B-lymphocytes, and occasional CD20-positive B-lymphocytes with larger atypical nuclei were found in both the subarachnoid space and the adjacent cerebellar cortex. There was mural and perivascular accentuation of the infiltrate, but vascular or tissue necrosis was not identified. In situ hybridization studies were negative for EBV. Polymerase chain reaction was suggestive of clonal gene rearrangement of the immunoglobulin heavy-chain gene. The overall histologic pattern was most compatible with the diagnosis of grade 1 lymphomatoid granulomatosis, even though the EBV studies were negative. The relative paucity of large B-cells might explain the absence of evidence for EBV because those are the cells that contain the virus in lymphomatoid granulomatosis. However, the presence of polymorphous cellular infiltrate composed of lymphocytes with smaller dark nuclei, histiocytes, atypical lymphoid cells with larger pale nuclei, and vascular mural and perivascular accentuation of the infiltrate was consistent with LG.
CT examinations of the neck, chest, abdomen, and pelvis demonstrated the presence of multiple pulmonary nodules, hypoattenuating lesions in both kidneys, and lymphadenopathy in multiple locations, including the mediastinum (precarinal and paraesophageal), renal hilum, retroperitoneum, and mesentery. Positron-emission tomography (PET) studies demonstrated hypermetabolic activity within the left renal hilar adenopathy (as well as within the mediastinal adenopathy and the pulmonary nodules). A laparotomy was performed with biopsies of the mesenteric and left renal hilar lymph nodes. The biopsy of the mesenteric lymph node showed only reactive hyperplasia, but the renal hilar lymph node contained an atypical lymphohistiocytic infiltrate similar to the lesion in the cerebellum but again without evidence for EBV on in situ hybridization.
Following the brain biopsy, the patient had approximately 2 weeks of ataxia, for which she needed a walker. She subsequently improved without medical therapy, and she currently walks independently. Her headaches have not recurred.
Follow-up cranial MR imaging at 4 months showed complete resolution of the smaller lesion, whereas the larger cerebellar lesion had significantly decreased in size. The lung nodules and abdominal lymphadenopathy also showed significant improvement on a thoracoabdominopelvic CT study performed 4 months after the initial presentation.
Discussion
Since the original description of lymphomatoid granulomatosis 35 years ago,7 this process has remained an enigma, with an elusive etiology.8 The presence of exuberant reactive T-cells led to the supposition that LG might be a T-cell lymphoproliferative disorder.8 However, recent studies1,8,9 have characterized LG as a type of EBV-positive B-cell lymphoproliferative disease with many similarities to posttransplant lymphoproliferative disorders. In almost all cases, the large B-cells are positive for EBV. However, the ability to demonstrate monoclonality correlates with histologic grade and the proportion of EBV-positive cells.8,10 In a few cases, the EBV-infected cells have been infrequent or undetectable, a finding that can be secondary to sampling error.8
Lesions of LG can be graded pathologically according to a previously reported system.11 Briefly, grade 1 lesions have a polymorphous angiocentric infiltrate without cellular atypia and with minimal-to-absent necrosis. EBV-positive cells are infrequent (<5/high-power field [HPF]) or absent. Grade 2 lesions contain large lymphoid cells with <20 EBV+ cells/HPF. Grade 3 lesions contain large atypical cells with >20 EBV+ cells/HPF. The pathologic findings in our patient were consistent with a grade 1 lesion. An additional finding in our patient was a low total level of IgG + IgA + IgM (491 mg/dL), suggestive of common variable immunodeficiency syndrome and considered to be a possible predisposition to LG.12
Jaffe and Wilson13 have reported spontaneous regression of grade 1 LG in some cases. Significant improvement in our patient's clinical condition without chemotherapy or radiation therapy during a 4-month period with almost complete resolution of some of the lung nodules, abdominal lymphadenopathy, and brain lesions also supports the histologic grading of the lesion.
CT and MR imaging findings of LG are nonspecific, most often consisting of multifocal intraparenchymal lesions within the supra- or infratentorial white matter; unifocal lesions are less common. These lesions can be solid with ringlike contrast enhancement and variable amounts of surrounding edema; alternatively, the lesions may appear as nonconfluent low-attenuation areas without enhancement.3
On MR imaging, the nontumorous LG lesions are nonspecific and mostly hyperintense on T2-weighted images and hypointense on T1-weighted images relative to brain parenchyma,1 possibly demonstrating ringlike, punctate, or linear contrast enhancement. The pattern of multiple punctate and linear enhancing lesions has been suggested as characteristic of LG.1 However, this enhancement pattern is not diagnostic because it can be seen in other vasculitic disorders and in demyelinating diseases.
Our patient presented with 2 diffusely contrast-enhancing lesions, unlike the previously described pattern of multiple punctate and linear enhancing lesions. Brain masses have been infrequently reported in patients with LG.1 Patsalides et al1 presented a total of 5 intracranial masses in 4 adult patients. In only one was a mass located in the cerebellar hemisphere, whereas the remainder was supratentorial. The literature to date documents only 2 pediatric cases of LG described on MR imaging, one presenting with bilateral parietal extra-axial masses4 and the other with diffusely increased T2 signal intensity of the cerebral white matter without a focal mass.6 To our knowledge, the present report represents the first pediatric patient with LG in whom the initial presentation was a cerebellar mass.
Diagnosis of this patient was difficult because initial presentation was with CNS symptoms and imaging studies showed a cerebellar mass. Therefore, our initial differential diagnosis suggested common pediatric cerebellar masses like medulloblastoma, astrocytoma, and ependymoma.14
LG and Wegener granulomatosis should be considered in the differential diagnosis of any pediatric patient presenting with CNS and lung symptoms. Autoimmune diseases, other systemic vasculitis, granulomatosis, and lymphoma should also be considered.5
This report describes an unusual occurrence of CNS involvement in lymphomatoid granulomatous in a 10-year-old girl. Despite its rarity, LG should be considered in the differential diagnosis of pediatric infratentorial mass lesions, especially in the presence of multiple lung nodules on chest CT and hypermetabolic lymph nodes on PET.
References
- Received March 5, 2007.
- Accepted after revision March 27, 2007.
- Copyright © American Society of Neuroradiology