Abstract
BACKGROUND AND PURPOSE: Structural MR imaging does not enable reliable differentiation of spinocerebellar ataxia (SCA) types 1 and 2 (SCA1 and SCA2), and imaging may be normal during the first years after the onset of symptoms. We aimed at determining whether measurements of the apparent diffusion coefficient (ADC) and fractional anisotropy (FA) may enable their differentiation.
MATERIALS AND METHODS: We enrolled 14 patients with SCA1, 11 with SCA2, and 9 age-matched controls. Diffusion tensor imaging (DTI) was performed on a 1.5T scanner, with b = 1000s/mm2 and 12 directions. ADC and FA were measured by means of regions of interest, positioned in the corticospinal tract at the level of the cerebral peduncle and at the level of the pons, in the transverse pontine fibers, in the superior and middle cerebellar peduncle, and in the hemispheric cerebellar white matter.
RESULTS: With respect to controls, the ADC was significantly elevated in the middle cerebellar peduncle and in hemispheric white matter in SCA1, and in all regions under consideration in SCA2. It was significantly higher in SCA2 than in SCA1 in all regions under consideration. With respect to controls, the FA was significantly reduced in all regions under consideration in SCA1 and in SCA2. It was significantly lower in SCA2 than in SCA1 in the transverse pontine fibers and in the corticospinal tract at the level of the cerebral peduncle. Correlations with clinical scores were found.
CONCLUSIONS: DTI did not enable differentiation between SCA1 and SCA2. However, strongly significant differences between the 2 subtypes and with respect to controls and correlations with clinical scores were found.
Spinocerebellar ataxias (SCAs) are autosomal dominant neurodegenerative diseases and are clinically and genetically heterogeneous. They are characterized by a range of neurologic symptoms including loss of balance and motor coordination, caused by progressive dysfunction of the cerebellum and of its afferent and efferent connections.1,2 The clinical diagnosis of specific subtypes is difficult because of the overlap of phenotypes among genetic subtypes, and the variability of clinical features found within distinct genetic subtypes.1
SCAs are caused by expansion of CAG triplet repeats in the coding region of the disease gene, resulting in production of a mutant protein with an abnormally long polyglutamine stretch.2,3 Since the discovery of the gene causing SCA1 in 1993, 27 loci for SCAs and the gene for dentatorubral and pallidoluysian atrophy (DRPLA) have been discovered.4,5
Different genetic entities can share common pathologic features in SCA1 and SCA2, including severe loss of Purkinje cells and neurons in the pontine and inferior olivary nuclei.6–9 SCA1 and SCA2 are the most commonly occurring types in Italy, accounting for 41% and 29% of cases, respectively. Worldwide, SCA3 is the most common genotype (21%), whereas SCA1 and SCA2 account for about 6% and 15% of cases, respectively.1,6 Microscopic examination reveals differences between the 2 types. In SCA1, although the degenerative process mostly affects the spinocerebellar system and the dentate nucleus, the Purkinje cells and the pontocerebellipetal system are relatively spared. In SCA2, degeneration of the cerebellar cortex and of the pontocerebellipetal system is marked, and the dentate nucleus is spared, though some fibrillary gliosis may be present.8–10 Although no treatment is available, from a clinical viewpoint differentiating the 2 subtypes is important for prognostic purposes, because SCA1 is characterized by more rapid progression than SCA2 and because SCA1 is often associated with heavier involvement of the pyramidal tract, whereas SCA2 is often associated with earlier onset of disturbances in eye movement.1
Neuroradiologic differentiation of SCA1 and SCA2 is difficult; furthermore, structural imaging may be normal during the first years after the onset of symptoms. The MR imaging correlates, namely atrophy and signal intensity abnormalities in the brain stem and cerebellum and supratentorial atrophy, can be assessed qualitatively, or with morphometric techniques.10–14 They appear relatively late in progression of the disease and have poor correlation with clinical disability.15
Mean diffusivity (also referred to as apparent diffusion coefficient, ADC) and fractional anisotropy (FA) from diffusion tensor imaging (DTI) have gained widespread acceptance as sensitive indicators to quantify microstructural damage of gray and white matter in neurodegenerative diseases.16,17 They have recently been used in the study of SCAs.10,15
We investigated changes in ADC and FA occurring in SCA1 and SCA2, with the purpose of determining whether they might enable differentiation between the 2 types; we also searched for significant correlations between ADC and FA and clinical scores.
Materials and Methods
Fourteen patients with SCA1 (4 female and 10 male, age 48.5 ± 11.3 years) and 11 patients with SCA2 (9 female and 2 male, age 42.4 ± 9.2 years) were scanned as part of a locally approved multicentric study.18 We obtained written informed consent from all participants, in accordance with institutional guidelines and with the Helsinki declaration.
The diagnosis was genetically confirmed by means of polymerase chain reaction of the gene expansions, as described elsewhere.6 On the same day of scanning, patients were assessed by a senior neurologist by means of the Scale for the Assessment and Rating of Ataxia (SARA), which quantifies the degree of neurologic impairment with a score ranging from 0 (no ataxia) to 40 (most severe ataxia).18
The clinical characteristics of the patients are given in Table 1; these were analogous to those seen in other series of patients with the same SCA genotypes.1,11,14,19–21 Nine age-matched controls (6 female and 3 male, age 48.2 ± 10.9 years), without a history of neurologic disturbances and with normal results on brain MR imaging were also enrolled.
Clinical features of patients with SCA1 and SCA2
Subjects were scanned on a 1.5T clinical MR imaging system. Structural imaging included axial proton-density (PD)/T2-weighted images (TR, 3500 ms; TE, 17 ms and 84 ms; 5 mm thick), coronal turbo spin-echo T2-weighted images (TR, 4100 ms; TE, 143 ms; 5 mm thick), and axial gradient-echo T2*-weighted images (TR, 700 ms; TE, 26 ms; 5 mm thick). Volumetric T1-weighted images were acquired by means of a magnetization-prepared gradient-echo sequence (160 sagittal sections; TR, 1640 ms; TE, 2.0 ms; isotropic voxel size, 1 mm3).
We performed DTI with a twice-refocused, single-shot, spin-echo echo-planar sequence, using 12 directions and b = 1000 s/mm2, with TR, 7500 ms; TE, 80 ms; NEX, 20; 192 × 256 matrix; FOV, 180 × 240 mm; thickness, 2.5 mm; no intersection gap.
Structural imaging was assessed jointly (ie, once per patient) by 2 senior neuroradiologists blinded to the identity of the patients, clinical data, and DTI measurements. The presence or absence of supratentorial, cerebellar, and pontine atrophy was assessed qualitatively by visual inspection of the volumetric T1-weighted images on the 3 planes, applying the same criteria normally used in clinical practice, namely evaluation of subarachnoid spaces surrounding the brain, the width of the sulci, the amplitude of the ventricles, and the relative size of the anatomic structures taken into consideration.11,22 The presence or absence of abnormalities of signal intensity in the basal ganglia, midline pontine fibers (of the raphe), and simultaneous presence of abnormalities in the midline and the transverse pontine fibers (“hot cross bun or cruciform sign”) was evaluated on T2-weighted images and on PD-weighted images, which are known to enable better delineation of areas of altered signal intensity.22,23 The 2 neuroradiologists agreed on the presence of atrophy and abnormalities in signal intensity in all of the cases.
We confirmed age matching with 2-tailed unpaired t tests among groups. Differences in SARA score, duration of disease, and CAG triplet repeats length between patients with SCA1 and patients with SCA2 were searched by means of 2-tailed unpaired t tests.
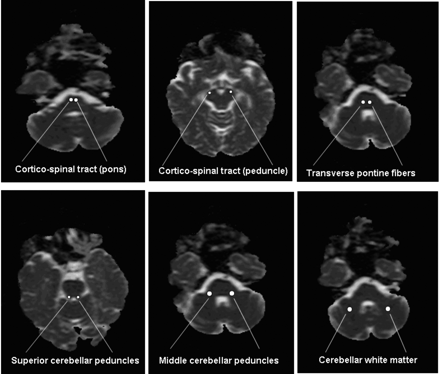
ADC and FA were measured with planar circular regions of interest (ROIs) positioned on the DTI color maps jointly (ie, once per patient), by 2 neuroradiologists blinded to patient identity, clinical data, and structural imaging. As shown in Fig 1, these were located bilaterally in the superior cerebellar peduncle, middle cerebellar peduncle, hemispheric cerebellar white matter, transverse pontine fibers at 2 levels, and in the corticospinal tract at the levels of the pons and cerebral peduncle. The ROI diameter ranged between 2 mm and 4 mm.
Positioning of ROI for ADC and FA measurements.
After confirming the absence of lateralization of ADC and FA changes with 2-tailed unpaired t tests separately for each ROI and group, we considered the measurements from the 2 hemispheres together. They were analyzed by means of 1-way ANOVA with group as factor, followed by the Tukey post hoc test. We searched for correlations between the SARA score and ADC and FA measurements by means of linear regression.
Results
No significant differences in age among the SCA1, SCA2, and control groups were found. No significant differences in duration of disease and SARA were found between the SCA1 and SCA2 groups. The CAG triplet repeats were significantly longer (P < .05) in SCA1 (47.1 ± 5.9) than in SCA2 (40.2 ± 2.9).
Supratentorial atrophy was present in 5 of 14 (36%) patients with SCA1 and in 5 of 11 (45%) patients with SCA2. Atrophy of the cerebellum was present in 14 of 14 (100%) patients with SCA1, but only in 5 of 11 (45%) of those with SCA2. Atrophy of the pons was seen in 3 of 14 (21%) patients with SCA1, and in 6 of 11 (54%) of those with SCA2. The transverse pontine fibers were hyperintense in 14 of 14 (100%) patients with SCA1, but only in 9 of 11 (82%) of those with SCA2. The cruciform sign was present in 3 of 14 (21%) patients with SCA1 and in 7 of 11 (64%) of those with SCA2. Abnormalities in signal intensity in the basal ganglia were not observed. Neither signal intensity abnormalities nor atrophy was detected in controls.
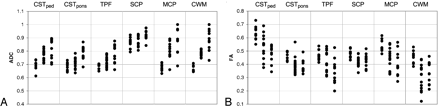
The results of ADC measurements are given in Table 2, and the corresponding scatterplot is shown in Fig 2A. The overall F value of the ANOVA was significant for all ROIs. With respect to control values, in SCA1 the ADC was elevated in the middle cerebellar peduncle and in hemispheric cerebellar white matter (P < .001); in SCA2 it was elevated in all regions under consideration (P < .001 for all regions under consideration, except P < .01 for the superior cerebellar peduncle). When compared with SCA1, in SCA2 the ADC was elevated in all regions under consideration (P < .001 for the corticospinal tract at the level of the pons, the transverse pontine fibers, and for hemispheric cerebellar white matter; P < .01 for the corticospinal tract at the level of the cerebral peduncle; and P < .05 for the superior and middle cerebellar peduncles).
Scatterplot of ADC (A) and FA (B) measurements. For each ROI, the columns are presented in the following order: controls, patients with SCA1, and patients with SCA2.
Results from post hoc comparisons for the apparent diffusion coefficient (ADC) between controls and patients with SCA1 and SCA2
The results of FA measurements are given in Table 3, and the corresponding scatterplot is shown in Fig 2B. The overall F value of the ANOVA was significant for all ROIs. With respect to control values, in SCA1 FA was reduced in all regions under consideration (P < .001 for hemispheric cerebellar white matter, P < .01 for the corticospinal tract at both levels, superior and middle cerebellar peduncles, and P < .05 for the transverse pontine fibers). Findings in SCA2 were analogous (P < .001 for all regions under consideration, except P < .01 for the corticospinal tract at the level of the pons, and P < .05 for the superior cerebellar peduncle). When compared with SCA1, in SCA2 FA was reduced in the transverse pontine fibers (P < .001) and in the corticospinal tract at the level of the cerebral peduncle (P < .05).
Results from post hoc comparisons for fractional anisotropy (FA) between controls and patients with SCA1 and SCA2
In SCA1, the SARA score was found to correlate with the ADC in the corticospinal tract at the level of the pons (r = 0.6, P < .05) and in the middle cerebellar peduncle (r = 0.6, P < .05). It was found to correlate with FA in the transverse pontine fibers (r = −0.6, P < .05), superior cerebellar peduncle (r = −0.7, P < .01), and middle cerebellar peduncle (r = −0.68, P < .01). In SCA2, it was found to correlate with the ADC in the corticospinal tract at the level of the pons (r = 0.7, P < .05), transverse pontine fibers (r = 0.8, P < .01), middle cerebellar peduncle (r = 0.73, P < .05), and hemispheric cerebellar white matter (r = 0.72, P < .01). It correlated with FA in the transverse pontine fibers (r = −0.67, P < .05) and in the middle cerebellar peduncle (r = −0.8, P < .01).
Discussion
Structural imaging highlighted a different pattern of degeneration of the cerebellum and pons in SCA1 and SCA2. In SCA1, atrophy of the cerebellum was observed much more frequently than atrophy of the pons. In SCA2, they occurred in a similar proportion of cases. Nevertheless, none of the structural imaging markers taken into consideration separated SCA1 and SCA2. Midline hyperintensity in the pontine base was seen in all patients with SCA1, in accordance with a previous report by Adachi et al.24 However, because it was also found in 80% of patients with SCA2, it cannot serve as a differential diagnosis. The “cruciform sign,” a typical feature of multiple system atrophy with cerebellar signs (MSA-C) reported to occur in advanced SCA2, was observed in 20% of patients with SCA1 as well.9 Although the degree of atrophy was not measured quantitatively, visual assessment as performed in clinical practice was judged adequate for the purpose of our study. Although this left a certain margin for rating subjectivity, we prevented biasing with blinding.
Adachi et al24 studied patients with SCA1, SCA3, and SCA6 but assessed DWI images qualitatively only, without measuring the ADC and FA. Della Nave et al15 compared SCA with other neurodegenerative diseases on the basis of ADC measurements but did not compare SCA1 and SCA2. Guerrini et al10 compared patients with SCA1 and SCA2 on the basis of measurements of the ADC in the pons, peridentate white matter, and middle cerebellar peduncle.
To our knowledge, this is the first diffusion-tensor study in SCA, measuring both the ADC and FA, and the second study comparing SCA1 and SCA2 on the basis of ADC measurements. Our results are essentially in agreement with those of Della Nave et al15 but are partially in contrast with those of Guerrini et al,10 who reported the absence of significant differences between the 2 subtypes. The reason for this discrepancy is unclear. Although the ages of the controls and the ages and duration of disease of patients with SCA1 were comparable, their group of SCA2 patients was characterized by considerably longer duration of disease and higher age.10 Furthermore, given the considerable difference in section thickness (6 mm in their study and 2.5 mm in ours), it is possible that partial voluming had a larger effect on their measurements.
The finding of increased ADC and reduced FA in the middle cerebellar peduncle and hemispheric cerebellar white matter, both in SCA1 and SCA2, is likely consequent to loss of Purkinje cells of the cerebellar cortex, which is known to be accompanied by extensive axonal loss, demyelinization, and reactive gliosis after degeneration of the corticodentate connections and pontocerebellipetal system.8,9
Increased ADC and reduced FA in the transverse pontine fibers in SCA2 is likely caused by fiber degeneration following marked neuronal loss in the pontine nuclei.8,25 The pronounced olivopontocerebellar atrophy occurring early in SCA2 may provide an explanation for the alterations in ADC and FA found in the transverse pontine fibers with respect to SCA1, in which brain stem involvement is milder.9
The finding of slightly increased ADC values in the superior cerebellar peduncle in SCA2 when compared with SCA1 is in line with the fact that SCA2 is characterized by disturbances in eye movement already in its early stage, whereas ophthalmoparesis occurs relatively late in SCA1.1 One may speculate that it is because of more pronounced degeneration of efferent projections from the dentate nucleus which merge toward the midbrain. However, this hypothesis is difficult to confirm because of the lack of neuropathologic studies comparing the involvement of the dentate nucleus in the 2 subtypes.8,9,26,27
With regard to the differences in ADC and FA in the corticospinal tract observed between SCA1 and SCA2, on the basis of measurements taken in the pons alone, one could speculate that they are simply the result of inclusion of the degenerating transverse pontine fibers in the ROI. However, this hypothesis is refuted by the fact that an equivalent pattern of alteration was also found at the level of the cerebral peduncle. Neuropathologic findings are controversial. In SCA2, the study by Iwabuchi et al found that the cerebral peducles were altered, but the study by Estrada et al excluded involvement of the corticospinal tract.8,9 The only study on SCA1 reported involvement in 2 of 8 patients.28 Involvement of the peripheral nervous system and sensory or sensorimotor neuropathy is found in about half of patients with SCA1, and in 80% of patients with SCA2.1 Motor-evoked potential with transcranial magnetic stimulation revealed abnormalities in 100% of patients with SCA1 but only in 18% of those with SCA2.29 Although our findings point to heavier involvement of the tract in SCA2, the exact cause of this apparent discrepancy with electrophysiologic findings cannot be elucidated on the basis of our findings alone.
A major limitation of our study was the small number of patients, and the results must be interpreted with caution as a consequence. Although the ADC values in the transverse pontine fibers and hemispheric cerebellar white matter correlated with the SARA score only in SCA2, the ADC in the corticospinal tract at the level of the pons and the middle cerebellar peduncle correlated with the SARA score, both in SCA1 and SCA2. Although FA in the superior cerebellar peduncle correlated with the SARA score in SCA1 only, FA in the transverse pontine fibers and middle cerebellar peduncle correlated with the SARA score, both in SCA1 and SCA2. Therefore, the middle cerebellar peduncle was the only location offering significant correlation with the SARA score, both in SCA1 and SCA2, and both for the ADC and FA. Another limitation of our study, and of other similar studies, on diffusion imaging in SCA was that interrater variability was not assessed.10,15
During the course of degenerative changes, the ADC and FA are generally inversely correlated: as the relative density of axonal membranes and myelin sheaths is reduced, diffusion becomes less anisotropic, and mean diffusivity increases.16 However, in our study we found a considerable decoupling between the results of ADC and FA comparisons. When considering this fact, one must take into account 2 main factors. First, one must consider that processes such as reactive gliosis may attenuate changes in the ADC, leaving FA comparisons more significant. Second, although the ADC is relatively homogeneous in the central nervous system, FA is not. As a consequence, notwithstanding the efforts to position the ROI in a repeatable manner, the marked atrophy occurring in SCAs may be a confounding variable affecting FA measurements more strongly than ADC measurements, potentially reducing the significance of comparisons by introducing additional variance.
Conclusions
Although the usefulness of DTI in differentiating patients with SCA1 and SCA2 and controls is limited because of the substantial overlap among the 3 groups, the finding of strongly significant differences and correlations with clinical scores suggests an important role in combination with structural imaging characterizing the phenotypes, and providing a quantitative measure of severity of disease.
Acknowledgments
This paper has become possible due to the contributions of my collegues Dr. Alberto Bizzi and Eng. Domenico Aquino for the optimization of the MR imaging sequences to whom I am deeply indebted and who I sincerely thank.
Footnotes
This work has been entirely self-financed, without any financial or material support from third parties.
References
- Received March 5, 2007.
- Accepted after revision April 25, 2007.
- Copyright © American Society of Neuroradiology