Abstract
SUMMARY: We present a case of a patient with systemic lupus erythematosus and secondary antiphospholipid syndrome. The patient presented with acute right cerebellar infarction and clinical and imaging evidence of brain stem and bilateral thalamic encephalopathy that resolved completely.
Antiphospholipid syndrome (APS) is a hypercoagulable state associated with antiphospholipid antibodies. Secondary APS is diagnosed when the findings of APS are associated with a systemic disease such as systemic lupus erythematosus (SLE). Stroke is a common central nervous system finding in these patients. Additional neuropsychiatric features such as epilepsy, headache, chorea, transverse myelitis, dementia, and depression have been described with APS.1 Although reversible encephalopathy affecting cerebral white matter is a well-described entity,2 few cases affecting the brain stem have been reported.3 Our patient demonstrated reversible deep brain encephalopathy in the setting of secondary APS. We describe this association with APS and review its diagnosis, pathogenesis, and radiologic features.
Case Summary
A 27-year-old woman with history of SLE, APS, hypertension, and diabetes mellitus presented with sepsis, hypotension with blood pressure of 79/49 mmHg, and osteomyelitis involving the sacrum. She had suffered multiple vascular complications related to APS, including stroke, deep venous thrombosis, and transverse myelitis with paraplegia, and she was being treated with coumadin and prednisone. Osteomyelitis was treated with IV vancomycin and piperacillin/tazobactam. During this course of treatment, she developed a rash, renal failure, and thrombocytopenia. Despite changing her antibiotic regimen to gatifloxacin, her symptoms progressed. Because this progression likely represented an exacerbation of her SLE, the steroid dosage was increased.
Three weeks after admission, the patient complained of headache and double vision. Neurologic examination revealed new horizontal diplopia, partial right sixth nerve palsy, and bilateral ptosis. An initial CT scan demonstrated hypoattenuation in the thalami and brain stem bilaterally. A low-attenuation area was present in the superior aspect of the right cerebellar hemisphere. The blood pressure at the time of the initial CT scan was 140/82 mmHg.
Acute infarct was suspected and MR imaging (MRI) and MR angiography were performed for further evaluation.
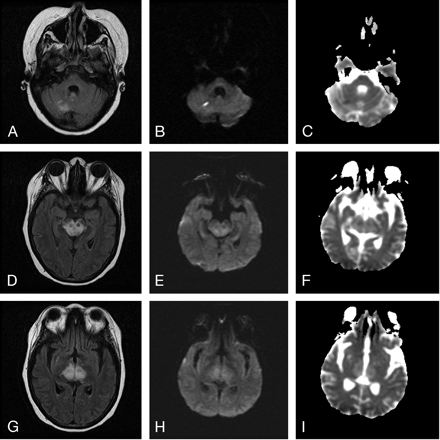
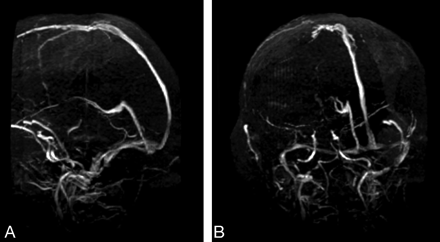
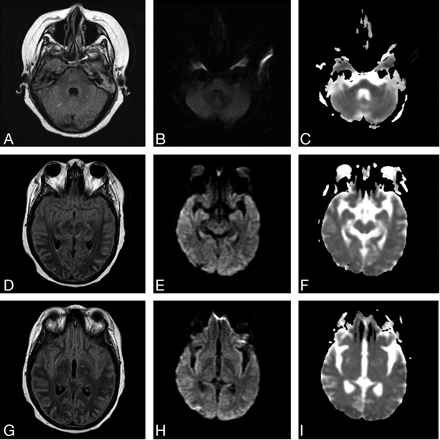
MRI demonstrated restricted diffusion in the superior aspect of the right cerebellar hemisphere, confirming acute infarction (Fig 1A–C). Abnormal hyperintense signal intensity was also seen within the brain stem and bilateral thalami (Fig 1D–I) with additional increased diffusion on the apparent diffusion coefficient map (Fig 1F, -I). At the time of initial MRI, the patient’s blood pressure was 144/84 mmHg. MR angiography of the intracranial and cervical vessels showed no abnormality. A CT scan 6 days later showed some resolution of abnormalities in the brain stem and thalami. MRI performed 7 days following the initial MRI confirmed partial resolution of abnormal hyperintense signal intensity in the thalami and brain stem. MR venography demonstrated no evidence of sinus or deep venous thrombosis (Fig 2A, -B). Two weeks after initial imaging, the right cerebellar infarct was resolving (Fig 3A–C), and the edema in the brain stem and bilateral thalami had resolved completely (Fig 3 D–I). The patient’s blood pressure was 100/47 mmHg at this time.
A–C, FLAIR (TR/TE, 9002/149.5), DWI (10000/105.7, b value of 1000), and ADC map images confirm the presence of acute right cerebellar infarct, D–I, FLAIR, DWI, and ADC map images show edema involving the brain stem and thalami.
A,B, Sagittal and oblique MR venography images (TR.TE, 40.0/5.3) show no evidence of sinus or deep vein thrombosis.
A–C, FLAIR (TR/TE, 9002/162.5) DWI (10000/95.2), and ADC map images show a resolving right cerebellar infarct. D–I, FLAIR, DWI, and ADC map images show resolution of the edema involving the brain stem and thalami.
The patient’s maximum creatinine level during the hospital stay was 2.2 mg/dL, and the creatinine level at admission and discharge was 1.5 mg/dL. The patient’s symptoms of headache and diplopia resolved within 2 days of presentation. However, her clinical course was complicated by respiratory failure. When her clinical status stabilized, she was discharged to a long-term care facility.
Discussion
APS includes the combination of antiphospholipid antibodies and hypercoagulability. Primary APS occurs in patients without clinical evidence of other autoimmune disease, whereas secondary APS occurs in association with diseases such as SLE. Studies have not shown major differences in outcome when comparing patients with primary APS and those with secondary APS associated with SLE.4
Several hypotheses have been advanced to explain the mechanism of thrombosis due to antiphospholipid antibodies. These include activation of endothelial cells, stimulation of oxidant-mediated damage of endothelium, interference with or modulation of coagulation proteins, and induction of thrombosis in areas of previous vascular injury.5 Clinical manifestations of thrombosis include stroke, myocardial infarction, deep venous thrombosis, pulmonary embolism, endocarditis, intrauterine growth retardation, and fetal loss.
The criteria for diagnosis of APS specify both clinical and laboratory abnormalities.6 Clinical criteria include vascular thrombosis in any soft tissue or organ1 or complications of pregnancy.2 Laboratory abnormalities may include elevated anticardiolipin antibody titers by immunoassay and detection of lupus anticoagulant by using coagulation assays. The only neurologic abnormality included in the diagnostic criteria is ischemic stroke. However, the association of APS with epilepsy, headache, chorea, transverse myelitis, amnesia, dementia, depression, and psychosis has been described.1 Pathogenesis for these neurologic syndromes in the setting of APS is not clear, but focal symptoms probably result from vascular lesions, whereas diffuse manifestations may be related to autoantibody or cytokine-mediated impairment of neuronal function.7
Central nervous system involvement in SLE is most often seen in the setting of APS with IgG type of anticardiolipin antibodies and with a previous history of thrombosis.8 Neurologic symptoms are much less common in SLE patients presenting with articular manifestations and discoid rash. Following an episode of thrombosis, risk of recurrence at the same site is high.
MRI findings described in patients with APS and SLE include a combination of infarcts and atrophy. Focal areas of infarct may be the most common finding in the brain.9 Because these MRI findings can mimic multiple sclerosis, the history of thrombosis and/or fetal loss, atypical location of brain lesions on MRI, and response to anticoagulant therapy are important diagnostic criteria.10
The clinical presentation and radiologic features of our patient are similar to those reported in cases of atypical posterior reversible encephalopathy syndrome (PRES).11–13 The radiographic findings can include abnormal hyperintense signal intensity on T2-weighted images involving the thalami, parts of the frontal and parietal lobes, the brain stem, subcortical white matter, and the internal capsule.11 The causes of PRES include hypertensive encephalopathy, eclampsia, and cyclosporine neurotoxicity. Additional risk factors include renal disease, SLE, and immunosuppression. The presence of increased diffusion in the thalami and brain stem in our patient indicates the presence of vasogenic edema and helps differentiate this patient’s disorder from metabolic processes such as central pontine myelinolysis, anoxic encephalopathy, and hypoglycemic encephalopathy, all of which induce cytotoxic edema.
Many cases in the literature describe an acute sustained rise in the diastolic blood pressure of greater than 100 in association with PRES.11, 12 In the present case, although the patient had a history of treated hypertension, she presented with hypotension and remained normotensive without treatment throughout the hospitalization, including the periods surrounding the imaging studies. The absence of severe hypertension, the extensive past history of APS, and the simultaneous finding of an acute right cerebellar stroke (a manifestation of APS) with reversible central encephalopathy suggest a complex pathophysiologic process. Additionally, encephalopathy, reversible edema, and involvement of the brain stem and cerebellum have been described in patients with SLE without a definite independent cause being identified.14 APS may place the patient at increased risk for developing PRES without hypertension or following minor perturbation of blood pressure. This mechanism could be due to transient ischemia or perhaps a direct effect of the antiphospholipid antibody causing increased vascular permeability or activation of vasoactive substances.
Reversible brain stem and thalamic encephalopathy can be considered among the many neurologic syndromes that occur in association with APS. Consideration of all manifestations of the syndrome will facilitate the diagnosis and enhance understanding of this protean disease, ultimately improving the care of affected patients.
References
- Received January 13, 2005.
- Accepted after revision December 8, 2005.
- Copyright © American Society of Neuroradiology