Abstract
SUMMARY: Subarachnoid hemorrhage following pituitary apoplexy is rare, and optic tract hemorrhage after the apoplexy is extremely rare. We report a case of optic tract hemorrhage after apoplexy that is not associated with hematologic disorders.
Hemorrhage within pituitary adenomas is relatively common.1 Rarely, hemorrhage following pituitary apoplexy expels into the subarachnoid space.2,3 Optic tract hemorrhage in pituitary apoplexy is extremely rare and has been reported only in a patient with idiopathic thrombocytopenic purpura.4 We report a case of optic pathway hemorrhage after pituitary apoplexy not associated with hematologic disorders.
Case Report
A 50-year-old man was admitted due to acute onset of blindness in the right eye. He had visual disturbance for 2 months and severe headache for 4 days before admission.
A pituitary macroadenoma was incidentally noticed on MR images 6 years before admission (not shown here). He had hypertension and was on medication for this for 7 years. He had no history of hematologic disorders.
On physical examination, the pupil was isocoric and light reflex was fixed in the right eye. The extraocular movement showed a full range. Sensory and motor function was intact. The patient’s right eye revealed no light perception and his left eye demonstrated decreased visual acuity with temporal hemianopia. Pituitary hormone evaluation revealed normal ranges. Platelet count, prothrombin time, and activated partial prothrombin time were within normal limits. The patient had no history of hematologic disorders.
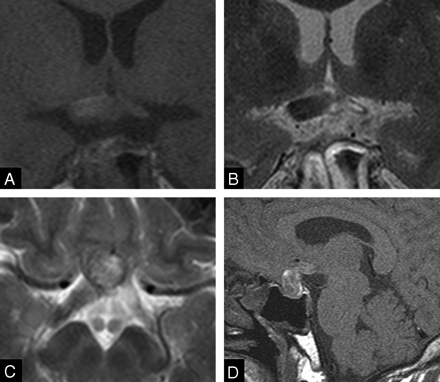
Orbit MR images on admission day 1 demonstrated mixed signal intensity prominence of the pituitary gland on T1- (Fig 1A) and T2-weighted images and mild contrast enhancement. The optic chiasm and optic tract showed no specific change of signal intensity or of size on T1- and T2-weighted images (Fig 1A–C). On admission day 2, T1-weighted coronal images revealed an edematous optic chiasm and right optic tract with hyperintensity on T1-weighted images (Fig 2A, -D). T2-weighted images showed hypointensity of the optic chiasm and right optic tract (Fig 2B, -C). The right optic tract increased in size within 1 day (Figs 1C, 2C). A pituitary mass showed mixed signal intensities on T1- and T2-weighted images. However, pituitary CT scans showed no evidence of subarachnoid hemorrhage.
MR images of patient on admission. T1-weighted coronal image (A) shows a hyperintense mass in the sellar and the suprasellar regions, suggestive of a subacute bleed. Note that neither the optic chiasm nor the optic tracts reveal evidence of hemorrhage on T1-weighted (A, B) or T2-weighted images (C).
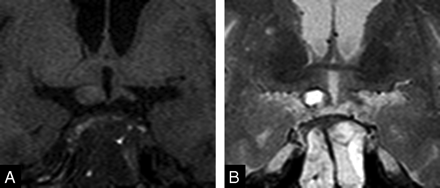
MR images of patient on admission day 2. Enlargement of the right optic tract with hyperintensity on T1-weighted (A) and hypointensity on T2-weighted images (B, C) is seen, suggestive of acute bleed. Note mild edema in the left optic tract. A sagittal T1-weighted image (D) reveals hyperintensity of the pituitary adenoma and right optic chiasm.
Transsphenoidal aspiration of the mass was performed on admission day 3. Necrotic and hemorrhagic adenoma was confirmed pathologically. On admission day 30, pituitary MR images demonstrated an optic chiasm, and the right optic tract showed isointensity on T1- and T2-weighted images (Fig 3 A, -B). Swelling of both optic tracts was decreased compared with MR images of admission days 1 or 2. Physical examination revealed faint light perception in the patient’s right eye. His left eye revealed improved visual acuity; however, temporal hemianopia was still evident.
T1-weighted (A) and T2-weighted (B) MR images on admission day 30. Optic tract swelling in the patient has decreased. Both optic tracts are revealed as isointensity on both images.
Discussion
In pituitary apoplexy, blood and necrotic tumor tissue are enclosed and compressed within the confined space of the sella. When the pressure gradient within the sella exceeds the resistance of the surrounding structures, blood is expelled into the subarachnoid space, producing a clinical picture that may be indistinguishable from aneurysmal subarachnoid hemorrhage.3
MR images in the present case showed that hemorrhage occurred acutely not only in the existing pituitary adenoma but also in the optic tract. Severe headache and acute visual loss supported MR imaging findings. Hemorrhage and edema increased on admission day 2. The precise mechanism of hemorrhage extending to the optic tract is unknown. The optic tract hemorrhage may have extended directly from the hemorrhagic adenoma or occurred primarily within an ischemic optic tract.4 In the present case, T2-weighted MR images on admission day 2 demonstrated different signal intensities of the optic tract from those of the hemorrhage of pituitary adenoma, suggesting that the optic tract hematoma occurred more recently than the pituitary hemorrhage. Recent reports have shown connections of Virchow spaces into the optic tracts that become edematous in patients with suprasellar masses.5 The connection of these subarachnoid spaces may be the route of spreading hemorrhage into the tract; alternatively, venous pressure increases may also be responsible for the spread of hemorrhage.
Optic tract thickening is commonly secondary to optic tract tumors such as gliomas, ependymoma, or primary CNS lymphoma,6 and it is also associated with sellar and suprasellar tumors including craniopharyngioma.7,8 Hemorrhage into the optic tract is extremely rare and has been reported only with pituitary apoplexy in the setting of idiopathic thrombocytopenic purpura4 and with a cavernous malformation within the optic tract.6
Transsphenoidal surgery for pituitary macroadenoma results in a progressive recovery of the visual field in 95% of patients.9 Even in apoplectic patients, early decompression of the pituitary mass leads to improvement in the visual deficit.10 However, in the present case, transsphenoidal aspiration was performed because the patient had experienced visual symptoms for 2 months. Delayed decompression may be related to the poor outcome of the vision in the patient.
Abrupt cranial neuropathy is also associated with pituitary apoplexy. Vision is most commonly disturbed; rarely third, fourth, or sixth cranial nerve palsies develop following pituitary apoplexy.11–13 Our patient showed visual disturbance for 2 months before admission and an abrupt decrease of the vision associated with acute hemorrhage of the optic tract.
In conclusion, we report a patient with acute hematoma of the optic tracts associated with pituitary apoplexy from a hemorrhagic pituitary adenoma. While rare, this finding is of interest in that ischemic or hemorrhagic complications of the optic tract with acute expansion of a pituitary adenoma may lead to visual loss that is not soley related to external compression of the optic pathways.
Early decompression of the pituitary adenomas is recommended for preservation or improvement of the vision.
Acknowledgments
The authors are grateful to Bonnie Hami, Department of Radiology, University Hospitals of Cleveland and Haaga Radiology Research, for her assistance in the preparation of this article.
References
- Received October 23, 2005.
- Accepted after revision December 2, 2005.
- Copyright © American Society of Neuroradiology