Abstract
BACKGROUND AND PURPOSE: The clinical outcome of acute necrotizing encephalopathy of childhood (ANEC), an encephalopathy characterized by symmetrical involvement of the thalami, has historically been poor, but recent studies have reported better outcomes. By devising a MR imaging scoring system, we determined the relationship between characteristic MR findings and clinical outcome of patients with ANEC.
METHODS: MR studies of 12 patients with ANEC were retrospectively reviewed. A MR imaging score was calculated for each patient according to the presence of hemorrhage, cavitation, and location of lesions. Clinical outcome of the patients was assessed, yielding outcome categories based on health state utility value. Spearman rank test was used to correlate the MR imaging score with clinical outcome of the patients.
RESULTS: Statistically significant correlation (r = 0.76, P = .001) was found between the MR score and the outcome category. The thalami were involved in all 12 patients, brain stem in 10, cerebral white matter in 8, and cerebellar white matter in 4. Hemorrhage was present in 5 patients and cavitation in 4. Clinical outcome category was 1 in 2 patients, 2 in 8 patients, and 3 in 2 patients. No patients were in category 4.
CONCLUSION: There is a significant and positive correlation between the clinical outcome and the MR imaging score in patients with ANEC. The relation between clinical outcome and each individual MR feature remains to be determined. Patients with ANEC may have a better clinical outcome than has been previously reported.
Acute necrotizing encephalopathy of childhood (ANEC) affects infants and children and is characterized by multiple, symmetrical lesions in the thalami, putamina, cerebral and cerebellar white matter, and brain stem tegmentum.1 More than 110 cases, predominantly found in Japan, Taiwan, and Korea, have been reported in the literature.1–5 Sporadic cases have been reported worldwide.6–9 The etiology and pathogenesis of this disease remain unknown. Although influenza A virus, mycoplasma, herpes simplex virus, and human herpes virus-6 have been reported as common causative agents,4 it is now believed that this disease is most likely immune-mediated or metabolic.3,10 It has been reported that cytokines, such as tumor necrosis factor receptor-1, interleukin-1, and interleukin-6, could mediate the disease.11,12 The clinical course of ANEC is fulminant, with a rapid onset of convulsions, impaired consciousness, vomiting, and variable degrees of hepatic dysfunction. Affected patients have high mortality and severe neurologic sequelae. From a pathologic perspective, the lesions show edema, petechial hemorrhage, and necrosis.1,3 Absence of inflammatory cells in affected brain parenchyma is characteristic, which differentiates this disease from the more common entities of acute disseminated encephalomyelitis and acute hemorrhagic encephalitis.
Neuroimaging features of ANEC are characterized by multiple, symmetrical lesions showing T2 prolongation in the thalami, frequently with accompanying lesions in the brain stem tegmentum, periventricular white matter, putamina, and cerebellum.3,4,13–15 The purpose of this study is to determine the relationship between MR imaging findings and clinical outcome of patients with ANEC.
Methods
Patients.
MR studies of 12 patients with ANEC, evaluated between 1996 and 2004, were retrospectively reviewed. The subjects consisted of 8 boys and 4 girls whose ages ranged from 7 months to 12 years (mean age, 3.87 years). Clinical features of the patients are summarized in Table 1. Diagnosis was based on the criteria of ANEC described by Mizuguchi et al3, acute encephalopathy with rapid conscious deterioration, absence of serum hyperammonemia, CSF pleocytosis, increase in CSF proteins, neuroimaging studies showing symmetrical, multifocal lesions involving the thalami, and the clinical absence of other diseases resembling ANEC.
Clinical features of 12 patients with acute necrotizing encephalopathy of childhood
MR Examination.
An initial and a follow-up MR study were performed on each of the patients. The mean interval between the onset of disease and the initial MR study was 5.2 days, and that between the onset of disease and the follow-up MR study was 62 days (Table 2). Although various MR pulse sequences with different orientations were obtained, axial T1-weighted spin-echo images, axial and coronal T2-weighted images, and axial and coronal postcontrast T1-weighted images were available for all patients. Axial or coronal fluid-attenuated inversion recovery (FLAIR) images were available for 7 patients.
Neuroimaging findings in the initial MR and the follow-up MR study in 12 patients with acute necrotizing encepalopathy of childhood
MR Imaging Evaluation.
Two neuroradiologists (A.M.W. and C.-H.T.) who were blinded to the results of outcome assessment independently reviewed all MR images. Each study was evaluated at least twice during separate sessions. In cases where there were different interpretations, the scans were re-evaluated, and a consensus was reached. An MR imaging scoring system was created subjectively to facilitate our appraisal of the imaging abnormalities. A MR imaging score (0–4) was calculated for each patient, based on both the initial and follow-up scans, according to a point system derived from the presence of hemorrhage, cavitation, and location of lesions, including the brain stem (Fig 1) and the white matter (cerebral, cerebellar, or both) (Figs 1 and 2). One point was awarded for each of these features. Because the thalami were, by definition, involved in all patients, their involvement was not scored in terms of location of lesions. Each study was evaluated at least twice in separate reading sessions. The presence of hemorrhage was defined as acute/subacute hematoma visualized on the initial study (Fig 2) and/or subacute/chronic hematoma on the follow-up study. Lesions with cavitation were defined as those having a sharply marginated border, with homogeneous and marked hypointensity on T1-weighted images and CSF-like intensity on T2-weighted images (Figs 2 and 3).
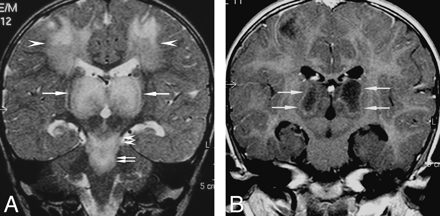
A 1.3-year-old boy (patient 1) left with spasticity and decerebrate posture. A, coronal T2-weighted imaging (4000 ms/90 ms, repetition time [TR]/echo time [TE]) shows symmetrical hyperintensity in the thalami (arrows), the centrum semiovale (arrowheads), and the brain stem, including the midbrain (double arrowheads) and the pons (double arrows). Note swelling of the thalami.
B, Postcontrast coronal T1-weighted imaging (630 ms/20 ms, TR/TE) shows irregular ringlike enhancement in the thalami (arrows).
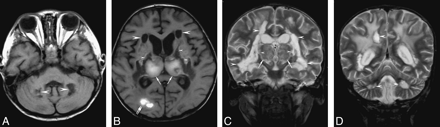
A 2.3-year-old boy (patient 12) with severe sequelae.
A, Axial T1-weighted imaging (449 ms/12 ms, TR/TE) shows sharply marginated hypointensity in the cerebellum (arrowheads).
B, Axial T1-weighted imaging shows hyperintensity in the thalami (arrows) and the right occipital lobe (double arrows). Sharply marginated hypointense areas are found in the cerebral white matter (arrowheads) and the internal capsules (small arrowheads). The lentiform nuclei also show mixed intensity (double arrowheads).
C and D, Coronal T2-weighted imaging show mixed intensity in the thalami (arrows), and hyperintensity in the cerebral white matter, internal capsules (small arrowheads), and the cerebellum (double arrowheads). There are 2 types of white matter lesions: those in the central white matter and internal capsules showing hyperintensity comparable with the CSF (arrowheads) and those in the subcortical regions being less hyperintense (double arrows).
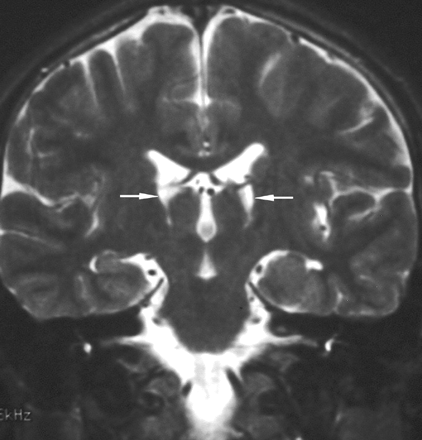
A 10-year-old boy (patient 5) recovered with hand tremor. Follow-up coronal T2-weighted imaging shows shrunken bilateral thalami with cavitation (arrows).
Outcome Assessment.
An experienced pediatric neurologist (H.-S.W.) who was blinded to the MR findings assessed the outcome of the patients based on data from medical records and telephone interviews. Duration of clinical follow-up was at least 18 months for each of the patients. The Health Utilities Index Mark 1 (HUI:1) proposed by Torrance et al,16 a 4-attribute health state classification system designed to uniquely categorize the health status of patients, was used for the assessment. The outcome of each patient was assessed according to 4 functions: (1) physical function (mobility and physical activity), (2) role function (self-care and role activity), (3) social and emotional function, and (4) health problems (general health and disease status). Each function was assigned a numerical value, from which an overall health state utility value (HSUV) was calculated. Four outcome categories were defined based on HSUV: good (G = 1.00 to 0.70), moderate (M = 0.69 to 0.30), poor (P = 0.29 to 0.00), and “worse than death” (W < 0.00).17 For the purpose of this study, each of these 4 categories was assigned a numerical value: good, 1; moderate, 2; poor, 3; and “worse than death,” 4.
Data Analysis.
The MR imaging score and the clinical outcome category of the patients were both analyzed as ordinal data. Spearman rank test was used for statistical analysis. Two calculations concerning the location of involvement were performed: one counted both the brain stem and white matter (calculation A) and the other counted only the brain stem (calculation B). A P value less than 0.01 (1-tailed) was considered statistically significant.
Results
Clinical outcome category was 1 in 2 patients, 2 in 8 patients, and 3 in 2 patients (Table 1)). No patients were in category 4.
Regarding the evaluation of the images, disagreement occurred in 1 of the 48 findings (2.1%).
Concerning the location of lesions, the thalami were involved in all 12 patients (100%), brain stem in 10 (83%), cerebral white matter in 8 (67%), and cerebellar white matter in 4 (33%). Hemorrhage was present in 5 patients (42%) and cavitation in 4 (33%) (Table 2)). Contrast enhancement was found in 8 patients (66%).
As the MR score increased, the outcome of the patients worsened. The patients having the highest MR scores were in the poor outcome category, whereas the patients with lower MR score (1 or 2) had better outcomes.
Calculation A.
Of the 4 patients with an MR score of 2, 3 were in clinical outcome category 2 and 1 was in category 1. The 3 patients with MR scores of 3 were in category 2. Of the 3 patients with an MR score of 1, the clinical outcome category was 2 in 2 patients and 1 in 1 patient. The 2 patients with an MR score of 4 were in clinical category 3.
Calculation B.
The 5 patients with an MR score of 2 were in outcome category 2. Of the 3 patients with an MR score of 1, the clinical category was 2 in 2 patients and 1 in 1 patient. The 2 patients with an MR score of 3 were all in outcome category 3. Of the 2 patients with an MR score of 0, the clinical category was 2 in 1 patient and 1 in the other patient.
Association between the MR score and the clinical outcome category was more significant in the calculation when counting only the brain stem as the location of involvement (calculation B) than that including all locations (calculation A), yielding r values of 0.76 (P = .001) and 0.67 (P = .001), respectively.
Discussion
In this study, we found a significant correlation between the MR imaging score and the clinical outcome in patients with ANEC. Our MR imaging score included the main features of ANEC, which have been reported as thalamic lesions that may bleed and/or cavitate.3,4 Hemorrhage and cavitation are destructive processes, causing mass effect on the brain and tissue loss, respectively. A recent study reported that hemorrhage and tissue loss were associated with a poor prognosis in ANEC.5 In our study, the distribution of lesions as well as the prevalence of hemorrhagic lesions and cavitation vary among patients. It has also been reported that patients with brain stem involvement tend to recover well.3 We thus developed a MR scoring system to include the main imaging features as well as the distribution of lesions and to correlate the results with clinical outcome. Nevertheless, it was not clear how to weigh the various features. Because we had no knowledge of the relative clinical significance of each feature, we finally opted to give a score of 1 to each feature. This method yielded a good correlation and was easy to compute.
Better clinical correlation was obtained in the calculation when counting only the brain stem as location of involvement. Relative to this finding, 2 possibilities arose: (1) the effect of white matter involvement and cavitation overlap because some white matter lesions cavitate1,3 and (2) involvement of cerebral white matter has less clinical impact than involvement of the brain stem. The distribution of extrathalamic lesions in our patients was more or less concordant with the few large series in the literature,1,4,5 with brain stem involvement in more than 85% of cases. However, the prevalence of hemorrhage and cavitation has not been well described in most reported cases. Kim et al5 reported a 57% hemorrhage rate and a 36% incidence of localized tissue loss in their series of 14 patients, findings comparable with our series.
The management of our patients was symptomatic and supportive; the combination of corticosteroids, anticonvulsants, and mannitol was the most frequently used regimen. Antiviral drugs, primarily acyclovir, were also administered to most of the patients on initial presentation, when viral encephalitis was suspected. However, the use of antiviral drugs was discontinued once the viral studies revealed no evidence of herpes simplex viral infection. We believe that such nonspecific use of antiviral drugs did not significantly affect the clinical outcome of patients.
The outcome of ANEC has been reported to be generally poor; approximately 65% of the affected patients died or were left with severe neurologic sequelae.1,4 Others have reported patients with good outcomes, suggesting a “mild” form of ANEC.8,18 In a series of 14 patients reported by Kim et al,5 there was no mortality, and approximately 60% of the patients were left with mild or no sequelae. Most of our patients fell into the moderate category (n = 8), with fewer patients in the good (n = 2) and poor (n = 2) categories, and with no mortality. We have encountered fatal cases but they did not have imaging studies that we could evaluate.4 Moreover, since 1996, ANEC has been increasingly recognized and thus detected earlier and more effectively managed. Therefore, we agree with Kim et al5 that the prognosis of ANEC has improved recently.
Before reaching the diagnosis of ANEC, a wide range of disorders affecting the cerebral deep gray matter should be considered. Symmetrical brain lesions are less frequently seen in hemolytic uremic syndrome, toxic encephalopathy, hemorrhagic shock, and encephalopathy syndrome.1 In addition, the clinical and laboratory findings in ANEC are different from the features in these diseases. Inborn errors of metabolism can be excluded based on biochemical studies. Hypoxic-ischemic encephalopathy may involve the deep gray nuclei bilaterally, but it is usually accompanied by a circulatory or hypoxic episode and an appropriate clinical course. Viral encephalitis may be difficult to exclude clinically. Certain encephalitides may also involve the deep gray matter symmetrically (eg, Japanese encephalitis). However, the thalamic involvement in Japanese encephalitis is not necessarily symmetrical, and involvement of the brain stem is uncommon.19 Moreover, the absence of inflammatory cells in affected brain parenchyma on neuropathology in ANEC is not compatible with inflammatory disorders such as acute hemorrhagic leukoencephalitis. Acute disseminated encephalomyelitis (ADEM) is a severe, postinfectious, inflammatory demyelinating disease. It is characterized on histologic examination by a diffuse perivenous lymphocytic inflammation with confluent demyelination.10 Gray matter can be involved, but to a lesser extent than white matter. The asymmetrical involvement and the response to steroid therapy in patients with ADEM may be helpful for differentiation. In a recent report comparing diffusion-weighted images of patients with ADEM and ANEC,20 decreased apparent diffusion coefficient (ADC) values were detected in ANEC but not in ADEM. Reye syndrome, although sharing many clinical similarities with ANEC, is more frequently associated with hyperammonemia, hypoglycemia, lactic academia, and mitochondrial deformation.21 Diarrhea, convulsion, and increased CSF protein are more common in ANEC than in Reye syndrome.3 The essential neuroradiologic finding in Reye syndrome is diffuse cerebral edema, though involvement of the thalami has been reported.22,23
Clinical symptoms and signs are nonspecific in ANEC; 40% of the patients have convulsions, 28% have impaired consciousness, and 20% have vomiting.3 These usually appear 12 to 72 hours after an antecedent illness. Coma may ensue by 24 hours. Blood biochemistry studies reveal elevated aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase. CSF study has been reported to show an increase in CSF protein but no cells, in two thirds of the patients.1,3,15 Motor deficits such as intention tremor, ataxia, speech impairment, choreoathetosis, spasticity, and focal neurologic signs, including hemiparesis and extraocular motility abnormalities, often develop in the chronic stage.
The most distinctive neuroimaging finding of ANEC is symmetrical, multifocal lesions that invariably involve the thalami.1–5,10 Other common locations of involvement are the brain stem tegmentum, cerebral white matter, internal capsule, putamen, and cerebellum. These lesions show low attenuation on CT and T1/T2 prolongation on MR studies. Ring contrast enhancement typically develops around the hemorrhagic areas by the second week of illness. Hemorrhage has been known to occur predominantly in the central portion of the involved deep gray matter but not in the cerebral white matter.1,10 In 5 of our patients with hemorrhagic thalamic lesions, 1 (patient 12) also had a hematoma in the right occipital lobe (Fig 2). No clinical evidence of trauma or coagulopathy was noted in this patient. Lesions in the deep gray matter are reported to consistently cavitate.1,10 However, in our series and the series reported by Kim et al5 in which the incidence of cavitation/localized tissue loss was well described, this occurred in less than half of the patients. In the 8 patients without cavitary lesions, the duration between initial and follow-up examinations was greater than 1 month in 5 patients, long enough for lesions to cavitate. The discrepancies between our results and those of earlier studies may be explained by the increased use of MR imaging, improved clinical management of recently encountered patients, and different definition of cavitation by various investigators. Reduced diffusion has been reported in the affected regions at the acute stage of disease.24,25
By devising and using the MR imaging scoring system, we provide a simple but effective way for both radiologists and clinicians to obtain preliminary information about the prognosis of patients with ANEC. Although the management of patients with ANEC is supportive,3,4 by identifying the patients with a potentially poor outcome based on pertinent imaging features and early use of newer, more aggressive treatment regimens, such as intravenous immune globulin,26 the clinical course and thus outcome of patients may be altered.
Our study is limited by the small sample size because ANEC is rarely encountered. Moreover, the interval between initial study and follow-up varied, ranging from 8 to 180 days. In addition, the age range of our patients is wide. Finally, the clinical outcome and MR imaging score may be oversimplified in our study. The ideal situation would be comparing each of the MR features with each outcome category, rather than computing a composite score. However, this would lead to fewer samples in each item for statistical evaluation, hampered further by the rarity of the disorder and our small sample size.
Conclusion
In ANEC, there is a significant and positive correlation between the clinical outcome and the MR imaging score, which is a composite of characteristic features including the presence of hemorrhage, cavitation, and location of lesions. Better correlation was obtained when only the brain stem was counted as location of involvement. The relation between clinical outcome and each individual MR feature remains to be determined. Patients with ANEC may have a better clinical outcome than has been previously reported.
References
- Received November 10, 2005.
- Accepted after revision January 5, 2006.
- Copyright © American Society of Neuroradiology