Abstract
SUMMARY: We report a patient with X-linked lymphoproliferative disease (XLP) who developed multiple central nervous system (CNS) manifestations of Epstein-Barr virus infection. XLP, or Duncan syndrome, is a rare inherited disorder characterized by the inability to clear Epstein-Barr virus infection. In addition to Epstein-Barr virus encephalitis, CNS lymphoproliferative disease, and lymphoma, this patient also developed MR angiographic evidence of diffuse fusiform aneurysmal dilation of intracranial vessels.
X-linked lymphoproliferative disease (XLP) is a genetic immunodeficiency in which patients display varied symptoms, including 3 primary phenotypes: fulminant infectious mononucleosis (46%), dysgammaglobulinemia (23.3%), and lymphoma (22.3%) (T. A. Seemayer, unpublished data, 2004). Epstein-Barr virus is known to cause central nervous system (CNS) complications such as encephalitis, lymphoma, and lymphoproliferative disorders. These Epstein-Barr virus–related complications usually occur in immune-deficient patients, for example, after organ transplantation or in HIV infection.1,2 We describe the constellation of CNS findings, including an unusual vasculopathy, in a patient with XLP.
Case Report
In March 1990, a previously healthy 7-year-old black boy was diagnosed with Burkitt lymphoma of the terminal ileum. Treatment with multiagent chemotherapy induced long-term remission. During a 10-year period, the patient developed recurrent sinus infections, an episode of neutropenia, herpes zoster infection, and pneumonia. The patient’s brother died at 5 years of age, secondary to large-cell immunoblastic T-cell lymphoma of the lungs.
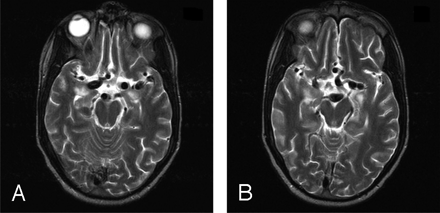
In July 2001, the patient presented with malaise, weight loss, night sweats, and fever. A complete blood count showed white blood count, 8600/mm3; hemoglobin level, 8.9 g/dL; and platelet count of 276 000/mm3, with atypical lymphocytes present on the smear. Epstein-Barr virus DNA was detected in the patient’s serum by polymerase chain reaction (>48 000 copies/mL). He developed lymphadenopathy, pleural effusions, arthralgias, and hepatitis. DNA analysis demonstrated a deletion of exon 1 of SH2D1A, the gene mutated in XLP. In September 2001, his short-term memory was severely impaired, and he experienced episodic disorientation and lethargy. His therapeutic regimen included intravenous immunoglobulins, acyclovir, and steroids. Brain MR imaging, performed on September 14, 2001, demonstrated dilation of multiple intracranial vessels (Fig 1), a midpontine lesion, and a 6-mm nonenhancing lesion in the right thalamus, which was hyperintense on T2-weighted images. CSF analysis revealed the following values: white cell count, 275/mm3 (90% lymphocytes [normal, 0–5]); protein level, 1080 mg/dL (normal, 10–40 mg/dL); and 6500 Epstein-Barr virus DNA copies/mL.
T2-weighted axial images demonstrate fusiform massively dilated bilateral ICAs, bilateral Al and Ml segments, and basilar artery.
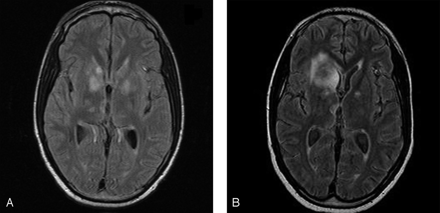
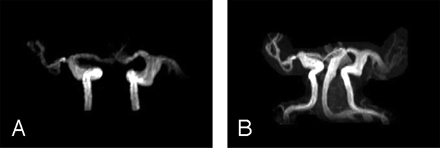
The patient was treated with cyclosporine, anti-CD20 monoclonal antibodies (rituximab), and anti-CD52 monoclonal antibodies (alemtuzumab). MR imaging, performed on October 15, 2001, showed stable dilated vasculature with interval development of T2-weighted hyperintense foci in the bilateral basal ganglia, internal capsules, thalami, and medial temporal lobes (Fig 2A), with minimal enhancement of the right caudate head. Punctuate foci were also noted in the cerebellar dentate nuclei. MR angiography characterized fusiform dilation of the bilateral cisternal A1, M1, and M2 segments of the anterior and middle cerebral arteries, supraclinoid segments of the internal carotid arteries (ICAs), and the distal portion of the basilar artery (Fig 3). Methylprednisolone (500 mg/day) was administered for 5 days to treat presumed CNS vasculitis. The patient showed mild clinical improvement with increased orientation, recognition, and recall. Repeat CSF analysis demonstrated improvement with a normal white cell count of 1/mm3 (93% lymphocytes), 249 mg/dL of protein, and 12 400 Epstein-Barr virus DNA copies/mL. However, subsequent CSF analyses showed recurrent pleocytosis, persistently elevated protein, and positive Epstein-Barr virus DNA. Flow cytometry of the CSF samples showed most lymphocytes to be mature reactive T-cells.
Axial fluid-attenuated inversion recovery images demonstrate multiple hyperintense foci of the bilateral basal ganglia and heads of caudate and (B) interval progression of the right basal ganglia lesions to involve the frontal lobe.
MR angiography maximum-intensity-projection images of the (A) anterior circulation and (B) combined anterior and posterior circulation demonstrate dilation of bilateral supraclinoid ICAs, Al, Ml, and M2 segments of the anterior and middle cerebral arteries, and (B) the basilar artery.
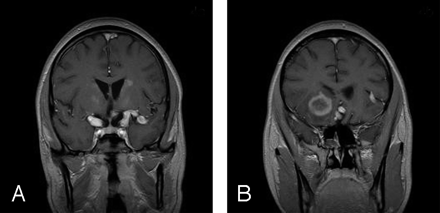
MR imaging, performed on November 9, 2000, revealed improvement of some basal ganglia lesions and interval development of T2-weighted hyperintense enhancing lesions in the bilateral frontal lobes and in the superior vermis (Fig 4A). Follow-up imaging, performed on November 29, 2001, showed significant increase in the size of the right frontal lobe enhancement, with development of central necrosis and minimal mass effect on the right frontal horn of the lateral ventricle (Figs 2B, 4B). His clinical condition was unchanged, with poor short-term memory and disorientation without focal weakness. He developed progressive cervical lymphadenopathy and cerebellar dysfunction. Biopsies of the enlarging right frontal lobe lesion and a cervical lymph node showed diffuse large B-cell immunoblastic lymphoma, which was Epstein-Barr virus–positive by in situ hybridization for Epstein-Barr encoded RNA (EBER).
T1-weighted postcontrast MR images obtained in the coronal plane show (A) enhancing foci within the bilateral lentiform nuclei, dilated supraclinoid segments of the bilateral ICAs and the left Ml segment of the middle cerebral artery; and (B) the peripherally enhancing right frontal lesion with central necrosis.
The patient was continued on steroids and started on hydroxyurea therapy. Multiple lung and liver nodules were detected by CT. On December 18, 2001, the enlarged right frontal brain lesion was surgically resected. Findings of pathology revealed lymphoma with extensive tumor necrosis and no normal brain parenchyma. The vessels present within the lymphoma were infiltrated by CD20-positive and EBER-positive lymphoma cells, with associated thrombosis and necrosis. Postoperative MR imaging, performed on December 28, 2001, revealed extensive edema around the operative sight, a new enhancing lesion in the left thalamus, and enlargement of lesions in the cerebellum and lateral aspect of the left frontal lobe. The fusiform dilation of intracranial vasculature remained stable throughout the terminal phase of the illness. The patient declined neurologically, developed hypoxia, and was transferred to the intensive care unit. On January 3, 2002, he received a maternal haploidentical stem cell transplant but developed hepatic dysfunction and venoclusive disease. In February 2002, he died. An autopsy was not performed.
Discussion
XLP is a genetic immunodeficiency affecting 1–2 in 106 males. Between 1980 and May 2002, the XLP registry reported 314 affected males. More than 75% of the currently known cases of XLP have been fatal, with fulminant infectious mononucleosis contributing the highest mortality rates (T. A. Seemayer, unpublished data, 2004). Complications of fulminant infectious mononucleosis include hepatic coma, respiratory distress syndrome, renal failure, and bone marrow failure. The development of lymphoma in XLP also portends a poor prognosis. XLP diagnosis has traditionally been made by fulfilling clinical diagnostic criteria, including 2 or more maternally related males manifesting one or more of the primary phenotypes.3 In 1998, the gene SH2D1A, which mutated in XLP, was identified. SH2D1A is localized to the long arm of the X chromosome in Xq25 and encodes a 128–amino acid adapter protein, sphingolipid activator protein, or signaling lymphocyte activation molecule–associated protein, which is involved in receptor signaling in T-cells and natural killer cells.2
Our patient developed multiple Epstein-Barr virus–related complications, which initially presented as an intractable infectious mononucleosis. CNS involvement began with increasing somnolence and short-term memory loss, suggesting the involvement of limbic structures. Encephalitis is a recognized complication of Epstein-Barr virus infection, especially in immune-deficient individuals. The diagnosis of encephalitis was confirmed by the presence of viral DNA and an increased number of white blood cells and protein in the CSF. Although consistent with the appearance of lymphoma in some regions, the varied signal intensity abnormalities seen on MR imaging may have represented multiple concurrent processes, specifically encephalitis. Basal ganglia involvement in Epstein-Barr virus encephalitis, with T2 hyperintensity as in our patient, is well described in the literature.4 In immunodeficient patients, the encephalitis is difficult to resolve, whereas immunocompetent patients are typically able to recover.5
Development of lymphoproliferative diseases and CNS lymphoma in association with Epstein-Barr virus infection is also well reported in the literature. In XLP, the ileocecal region is the predominant location of primary lymphoma, thought to be due to the faulty B-cell immune response within Peyer patches.1 The terminal phase of our patient’s illness was characterized by development of B-cell lymphoma, histologically confirmed in the brain and lymph nodes. Although CNS lymphoma, as seen in our patient, is uncommon in XLP, it is well described in other immunodeficiency disorders such as Wiskott-Aldrich syndrome and AIDS. The privileged immunologic status of the CNS may contribute to the occurrence of CNS lymphoma by allowing altered lymphoid cells to localize and proliferate. Although immunocompetent patients with primary CNS lymphoma have a 5-year survival rate of up to 60%, immunodeficient patients with Epstein-Barr virus–related CNS lymphoma fare much worse.6,7
Development of diffuse fusiform aneurysms in a patient with XLP is unusual, and to our knowledge, imaging findings in this entity have not been previously published. Three cases of XLP-associated vasculitis have been reported in the XLP registry. In a case report from 1986, the postmortem findings demonstrated medium and small-vessel arteritis of the cerebral hemispheres, pons, and dura, associated with microscopic aneurysmal dilation and fibrinoid necrosis.8 In another case report from 1983, postmortem findings revealed necrotizing vasculitis of various small- and medium-sized muscular arteries including the meninges, right cerebrum, and spinal cord. A single berry aneurysm was demonstrated by angiography, and histologic patterns involved aneurysmal dilation, irregularly thickened intima, and interrupted elastic lamina. Damaged small and medium arteries contained lymphocytic infiltration of all 3 layers, predominantly within the tunica intima and adventitia.9 Our patient may have developed similar pathology because blood vessels within the tumor were extensively infiltrated by lymphoma cells and, therefore, could not be evaluated for inflammation. No normal brain tissue was available for histologic evaluation of blood vessels to confirm vasculitis. The involvement of intracranial vessels in the subarachnoid space suggests that the combined exposure to virus in the blood and surrounding CSF may have predisposed our patient to this unusual vasculopathy.
CNS pathology in Epstein-Barr virus infection is considered to involve direct CNS invasion, infiltration of Epstein-Barr virus–infected lymphocytes, and deposition of antibody-antigen complexes in the endothelium, causing disruption.10 These mechanisms, as well as the direct infection of blood vessels, may be responsible for the vasculopathy seen with Epstein-Barr virus infection. Intracranial aneurysm etiologies include atherosclerosis, hypertension, vasculopathy, trauma, drug abuse, neoplasm, and infection. Large-vessel arteritis, with aneurysmal dilation, has been described in chronic Epstein-Barr virus infection, and common histologic patterns are seen in patients with and without XLP.11 Infectious etiologies, including Epstein-Barr virus, may contribute significantly to aneurysm formation, particularly in immunocompromised patients. Although fusiform aneurysms are rare, <1% of intracranial aneurysms, they have been increasingly reported in association with AIDS, suggesting infectious causes.12
Conclusion
Multiple CNS manifestations of chronic Epstein-Barr virus infection may occur in immunodeficient patients. Our patient developed an unusual vasculopathy manifest as diffuse fusiform dilation of CSF–bathed blood vessels. Systemic exposure to Epstein-Barr virus and additional exposure through the CSF may have contributed to the development of these aneurysms.
Footnotes
Presented as an Excerpta Extraordinaire at the 43rd annual meeting of the American Society of Neuroradiology, Toronto, Ont, Canada, April-May 2005.
References
- Received March 17, 2005.
- Accepted after revision May 17, 2005.
- Copyright © American Society of Neuroradiology