Abstract
BACKGROUND/PURPOSE: Identification of carotid near-occlusion is essential before calculation of percent stenosis because stroke risk is lower than other severe stenosis and the treatment benefit is less. Calculations with reduced distal diameters are fallacious. CT angiography (CTA) is convenient and accurately quantifies internal carotid artery (ICA) stenosis.
METHODS: In a blinded protocol, 268 carotid artery CTAs for known or suspected carotid disease were independently evaluated by 2 neuroradiologists. All carotid arteries were measured in millimeters at the narrowest diameter of the stenotic bulb, distal ICA well beyond the tapering bulb, and distal external carotid artery (ECA). Near-occlusions were independently identified, with disagreements settled by consensus meeting. Receiver operating characteristic (ROC) curve analysis defined the threshold values that best predicted near-occlusion according to (1) ICA stenosis, (2) distal ICA, (3) distal ICA: contralateral distal ICA, and (4) distal ICA: ECA. Paired permutations of variables were evaluated.
RESULTS: Forty-two near-occlusion distal ICAs were identified. The ROC-derived threshold values determined near-occlusion carotid stenosis with a sensitivity range, 90.2–97.3; specificity, 84.1–89.9; positive predictive value (PPV), 61.3–66.7; and negative predictive value (NPV), 96.7–99.4. Ranges for paired permutations were also determined: sensitivity, 82.9–91.9; specificity, 95.4–96.8; PPV, 78.6–85.7; and NPV, 96.3–98.4.
CONCLUSIONS: Threshold values provide guidelines for CTA interpretation when assessing carotid artery disease and the presence of near-occlusion. Ultimate identification of near-occlusion requires the interpreter’s judgment, with attention to the following criteria: (1) notable stenosis of the ICA bulb and (2) distal ICA caliber reduction compared with (A) expected size, (B) contralateral ICA, and (C) ipsilateral ECA. Near-occlusion distal ICAs can be reliably identified on CTA.
Identification of near-occlusion stenosis with associated decreased diameter of the distal internal carotid artery (ICA) is essential for proper diagnosis and management of atherosclerotic carotid artery disease. Ratio calculations to determine percent degree of carotid bulb stenosis, as in North American Symptomatic Carotid Endarterectomy Trial (NASCET) and other study methods,1-4 rely upon proper measurement of the distal ICA diameter for the denominator data. NASCET methods required that no ratio calculation be done in cases of collapsed or partly collapsed distal ICAs above a severe ICA bulb stenosis,1-5 because the use of this denominator data would provide a fallacious stenosis calculation that underestimates the true stenosis.4-7 A collapsed distal ICA was defined as an obvious threadlike lumen, often referred to as the “string sign.” A partly collapsed distal ICA was defined as a narrowed vessel with the appearance of a small, otherwise normal, artery.
Identification of near-occlusion affects management of atherosclerotic carotid artery disease. The primary management choices include a medical management approach (lifestyle, exercise, pharmaceuticals, etc) versus a revascularization approach (endarterectomy, carotid stent placement). Although revascularization via carotid stent placement is increasingly common, the large randomized outcome studies of carotid artery stenotic disease systematically evaluated the risks and benefits of carotid endarterectomy.1-3 The NASCET data showed that carotid endarterectomy is highly beneficial in symptomatic patients with ≥70% stenosis,1 but only in cases without near-occlusion stenosis.3,5,8-10
A recent review of catheter angiograms in patients with severe ICA stenosis (NASCET style ≥70%) from the NASCET and European Carotid Surgery Trial (ECST) was performed to estimate the accuracy of near-occlusion identification and to assess prognosis for patients with near-occlusion.5 Near-occlusion was defined as any decrease in the expected diameter of the distal ICA above a severe stenosis. This expands the catchment of “near-occlusion” to include cases with far less reduction of distal ICA diameter than would be called “collapse.” Most cases in this review showed subtle degrees of distal ICA decrease requiring a consistent high index of suspicion and systematic image evaluation.5
Despite their pooled data from the NASCET and the ECST studies, the relatively small sample size for near-occlusion, combined with low stroke event rates,5 did not allow determination of any statistically significant conclusion supporting the use of endarterectomy for near-occlusion cases. The authors concluded that it is reasonable to consider endarterectomy in patients with near-occlusion ICA stenosis with distal ICA caliber reduction.5 However, the benefit of endarterectomy for near-occlusion patients will be muted, even for cases with “subtle” near-occlusion, in comparison to patients with severe stenosis and normal-sized distal ICAs.5 These results complement prior studies in the literature, showing a lower risk of ipsilateral stroke in patients with near-occlusion stenosis in comparison to patients with severe stenosis and a normal distal ICA diameter.3,9-10 Decisions regarding revascularization of near-occlusion need to be made with appropriate relative risk: benefit considerations. The risk: benefit considerations of near-occlusion are different from those for severe stenosis without near-occlusion, as shown in endarterectomy trials.1,3
Identification of the well-recognized threadlike appearance of the so-called string sign or major distal ICA collapse associated with a near-occlusion stenosis is straightforward (Fig 1). When near-occlusion cases are more subtle, detailed attention is focused on not only the ICA in question, but also the surrounding vessels5 (Figs 2 and 3). In the series of cases reported from combined NASCET and ECST data,5 262 patients (n = 1216) were identified as having near-occlusion. However, only 16 of these cases had distal ICA lumen collapsed enough to be called “string sign.”
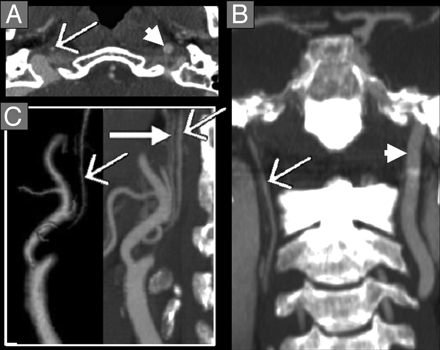
Near-occlusion ICA stenosis with distal ICA collapse, threadlike “string sign.” CTA axial source image (A) near the skull base and coronal MPR (B) showing collapse of the right distal ICA (thin arrow) in comparison to normal caliber left distal ICA (arrowhead). These arteries are continuous from the proximal carotid bulb and are headed toward the carotid canal. Both features should be identified on MPRs with reference to the axial source images to distinguish collapsed ICAs from other vessels, especially the ascending pharyngeal artery. C, 3D-rendered (left) and oblique sagittal MPR (right) of the right carotid arteries showing the severe carotid bulb stenosis, appearing amputated on reformatted images. There is collapse of the right distal ICA (thin arrow), similar in size to the ascending pharyngeal artery on the oblique sagittal MPR (thick solid arrow). The identification of both these vessels was confirmed on the axial source images and other MPRs by identifying their origins and anatomic continuations.
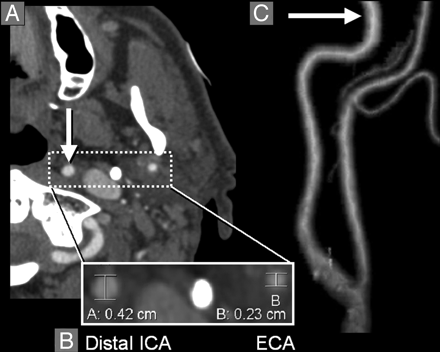
Normal distal ICA. A, Axial CTA image at the level of the left distal ICA (arrow) and distal ECA (both enclosed by dashed-line box). Densely calcified styloid process is between the ICA and the ECA. B, Magnification of the distal ICA and distal ECA with diameter measurements; the distal ICA diameter (A, 0.42 cm) is substantially larger than the distal ECA diameter (B, 0.23 cm). Densely calcified styloid process is between the ICA and the ECA. C, 3D-rendered image of the left distal ICA and distal ECA, showing their normal relationship (arrow, distal ICA).
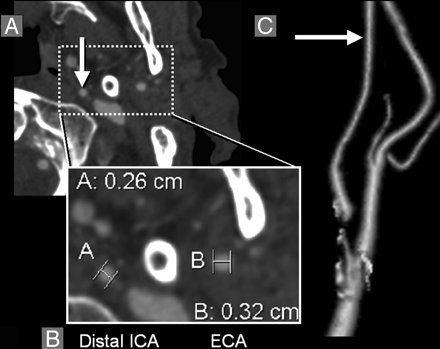
Decreased diameter of distal ICA lumen associated with near-occlusion stenosis. A, Axial CTA image at the level of the left distal ICA (arrow) and distal ECA (both enclosed by dashed-line box). B, Magnification of the distal ICA and distal ECA with diameter measurements; in this case, the distal ICA diameter (B, 0.26 cm) is smaller than the distal ECA diameter (A, 0.32 cm). C, 3D-rendered image of the left distal ICA and distal ECA, showing the decreased distal ICA (arrow, distal ICA), secondary to near-occlusion ICA stenosis in this case. Distal ICA reduction that is near equal in diameter to distal ECA is substantially decreased from expected normal as shown in Fig 2). The maximum left ICA bulb stenosis measured 0.07 cm, shown on 3D-rendered image as an amputated segment. Calcification surrounds segments of the left carotid bifurcation.
Criteria for detecting near-occlusion are systemically outlined in a recent re-evaluation of 1216 catheter angiograms of severe stenosis cases from the NASCET and ECST studies.5 We have adapted these criteria as they apply to CT angiography (CTA), hypothesizing that abnormal distal ICAs above near-occlusion stenoses can be reliably identified by using quantitative data from CTA.
Methods
Patients/Subjects
Examinations were retrospectively collected from a single institution, by using an AGFA Impax 4.5 PACS data base (Mortsel, Belgium) from August 2003 through March 2004. Examinations were entered into the study for all consecutive patients examined during this time period with the history of known or suspected carotid artery disease. Examinations were not included for cases of trauma, dissection, vascular anomaly/malformation, pre/postoperative studies unrelated to carotid atherosclerotic disease, cases primarily evaluating the posterior circulation, inadequate coverage, and/or technical errors precluding full evaluation of the cervical carotid arteries. The study was approved by our center’s research ethics board (project identification number 411-2004). Informed consent was not required for inclusion in this study and its evaluation of records and images.
Materials/Image Acquisition
All CTA examinations were performed by using a GE Medical Systems (Waukesha, Wisc) Lightspeed Plus 4-section helical CT with a 6.3-MHU Performix tube. Images were obtained from C6 to vertex by using the helical HS mode with 7.5 mm/rotation and 1.25 × 1.25 mm collimation (120 kVp, 350 mA). Intravenous access was via an antecubital vein by using an 18- or 20-gauge angiocatheter. A total of 100–125 mL Omnipaque 300 were injected at a rate of 4.0 to 4.5 mL/s, with a 17-second delay or the use of Smart Prep at the pulmonary artery.
The CT technologists performed all the postprocessing multiplanar reformats (MPRs) at the CT operator’s console. Coronal and sagittal MPR images were created at 10.0-mm thickness, with 3-mm intersection gaps. Bilateral rotational MPRs were created at the carotid bifurcations with a thickness of 7 mm and spacing by 3 mm. 3D-rendered images were created on a GE Advantage Workstation for selected patients by CT technologists. All images were viewed on AGFA Impax 4.5 PACS workstations.
Image Analysis/Interpretation
All cases meeting the inclusion criteria were independently evaluated by 2 neuroradiologists in a blinded protocol. Near-occlusion stenoses with abnormal distal ICAs were identified by adapting recently published conventional angiography criteria for identifying subtle findings of near-occlusion5 to CTA. Specifically, the identification of a near-occlusion was based upon evaluation of the following criteria: (1) notable stenosis of the ICA bulb; (2) distal ICA caliber reduction with comparison to (A) its expected lumen size (Figs 1–3), (B) the contralateral ICA lumen (Fig 1), and (C) the ipsilateral external carotid artery (ECA) lumen (Figs 2, 3). All interpretative disagreements were further reviewed at a consensus meeting, randomized with an assortment of severely stenotic carotid arteries (NASCET ratio ≥70%) without near-occlusion and with near-occlusion distal ICAs (taken from the sample of agreed upon cases).
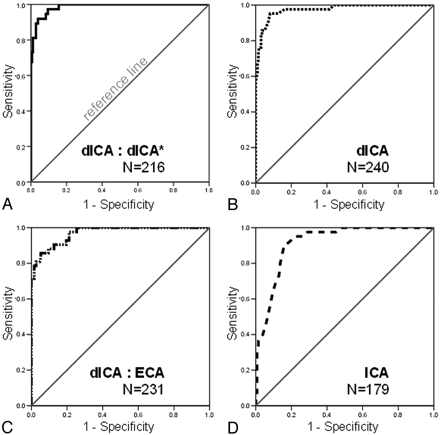
ROC curves depicting the performance of each model in identifying near-occlusion stenosis. The model is more accurate the further the curve lies above the reference line (greatest area under the ROC curve). A, Ratio of the distal ICA lumen to the contralateral distal ICA lumen (dICA:dICA*, solid line), the most accurate model to identify near-occlusion stenosis. B, Distal ICA lumen (dICA, dotted line) is the second most accurate model. C, Ratio of the distal ICA lumen to that of the ipsilateral ECA (ICA: ECA, dot-dashed line), is the third most accurate model. D, The ICA stenosis model (ICA, dashed line) is the least accurate to identify near-occlusion stenosis.
Millimeter measurements were obtained by using the submillimeter measurement and magnification tools on the PACS workstation, as described elsewhere11 for quantification of carotid arteries with CTA. All measurements were obtained from the axial source data. Carotid stenosis measurements were obtained at the narrowest stenotic portion of the carotid bulb. Although it is recognized that ICA stenosis may exist beyond the bulb, only maximum stenosis of the bulb region was considered for the stenosis measurement, paralleling the methods from our catheter angiographic model.5 The distal ICA was measured well beyond the bulb where the walls are parallel and no longer tapering from the carotid bulb as per NASCET.2,4 Distal ECA diameter was measured before its terminal bifurcation, at a similar axial level to the distal ICA measurement. MPRs identified the carotid orientation to ensure true cross-sectional measurements in all of the evaluated arteries.11 Arteries oblique to the axial plane were measured perpendicular to their oblique axis. These measurements were verified with measures from MPRs to ensure accuracy in obtaining the narrowest diameter in a true cross-sectional plane.
Statistical Methods
All raw data were analyzed by using the statistical software package, SPSS for Windows (version 12.0.0; SPSS, Inc., Chicago, Ill). A P value <.01 was considered to indicate a statistically significant difference. All missing data were excluded from calculations in a pairwise fashion.
Correlation coefficients (Pearson product moment) were calculated with 2-tailed significance to evaluate interobserver agreement for all millimeter measurements.
Receiver operating characteristic (ROC) curves provided a visual comparison of each model’s accuracy in defining near-occlusion stenosis and its associated distal ICA diameter reduction. These 4 measurement models were based upon criteria identifying the findings of subtle presentation of near-occlusion as outlined above5 and adapted to CTA. These models are based upon the quantitative evaluation (millimeter measurements) of these variables: (1) ICA bulb stenosis, (2) distal ICA lumen, (3) ratio of the distal ICA lumen to the contralateral distal ICA lumen (dICA: dICA*), and (4) ratio of the distal ICA lumen to that of the ipsilateral ECA lumen (dICA: ECA). Cases of recognized bilateral near-occlusion and of total occlusion were excluded from ratio calculations listed above, because such calculations would be fallacious.
Threshold values were assigned for each model by using the ROC-curve analysis to maximize the sensitivity and specificity of the threshold value. Contingency tables tested the paired permutations of the independent models, based upon the defined threshold values. Positive and negative predictive values were calculated for all data.
Results
Image Analysis
Each of 2 neuroradiology reviewers independently evaluated 268 carotid arteries (134 CTA cases). Of the 268 carotid arteries, 42 (15.7%) were classified as true near-occlusions, selected independent of clinical information. In the initial review, the 2 independent neuroradiology observers agreed on 36 near-occlusion carotid arteries and disagreed on 13 others. These initial agreements and disagreements (total of 49 carotids) were combined with a random assortment of another 105 severely stenotic carotids, for a total of 154 randomized carotids. These carotids were reviewed at a consensus conference, which classified a total of 42 carotids as near-occlusions without any disagreements. There were no cases of recognized bilateral near-occlusion. Three of the 42 near-occlusion carotid arteries had contralateral total occlusion.
Interobserver variability for all millimeter measurements was excellent. The Pearson product moment correlation coefficient for the carotid stenosis was 0.81 (n = 205), the distal ICA was 0.91 (n = 260), and the distal ECA was 0.71 (n = 234). Because the variability between the observers was minimal, the data pairs between the reviewers were averaged to create a mean millimeter measurement for each specific vessel. These mean data were used in the ROC curve analysis and validity measurements.
ROC Curve/Statistical Analysis
ROC curves tested each model’s ability to correctly identify the 42 identified near-occlusion carotid arteries. The most accurate test model in defining the presence of near-occlusion is the ratio of the distal ICA to the contralateral distal ICA (dICA: dICA*), as evidenced by the greatest area under the ROC curve at 0.986 (95% confidence interval [CI] = 0.974–0.999; n = 216; Fig 4A). The distal ICA ratio threshold value that best predicts near-occlusion stenosis with the greatest sensitivity and specificity is 0.87 (Table 1). Therefore, any distal ICA that is equal to or less than 87% of the size of its contralateral distal ICA, in the setting of ipsilateral severe carotid bulb stenosis, is considered to be a near-occlusion distal ICA.
Validity statistics of near occlusion single-variable test models (in order of decreasing accuracy)
The second most accurate test model to evaluate for near-occlusion is the measurement of the distal ICA itself. The area under the ROC curve for the distal ICA is 0.975 (95% CI = 0.952–0.997; n = 240; Fig 4B). The distal ICA threshold measurement with the greatest sensitivity and specificity in detecting near-occlusion stenosis, is ≤3.6 (Table 1).
The ratio of the distal ICA to the ipsilateral distal ECA (dICA: ECA) is the third-most-accurate test model to determine near-occlusion stenosis, with an area under the ROC curve at 0.970 (95% CI = 0.947–0.992; n = 231; Fig 4C). The threshold value for this variable is a ratio ≤ 1.27 (Table 1).
The least-effective single test model to determine near-occlusion stenosis is the ICA stenosis. The area under this ROC curve is 0.918 (95% CI = 0.878–0.959; n = 179; Fig 4D). The threshold for this variable is a carotid bulb stenosis of ≤1.3 mm (Table 1).
Specificity is improved by combining the single test model variables into paired permutations. Each case is then required to meet the threshold values of both the paired single test models. Cases that meet the threshold values for both the distal ICA test model and the distal ICA ratio test model (dICA:dICA*), have the highest sensitivity and specificity of all the paired test models (sensitivity = 91.9%; specificity = 96.0; n = 235) (Table 2).
Validity statistics for the paired permutations of near occlusion single-variable test models (in order of decreasing accuracy)
A summary of the combined test results is contained in Table 2, in descending order of accuracy, for the detection of near-occlusion stenosis. As expected, the most robust combinations are those including the highest performing single test models, the ratio of the distal ICAs (dICA:dICA*) and the ipsilateral distal ICA measurement.
Discussion
Proper diagnosis and management of atherosclerotic carotid artery disease requires a multifaceted approach. Clinically, the presence and characterization of symptoms from a carotid source must be considered. Diagnostically, the presence of a significant ICA stenosis and the characterization of that stenosis must be considered.
The ICA, however, should not be evaluated in isolation. Decisions regarding potential revascularization should always consider the possibility of ipsilateral near-occlusion. Multiple studies have proved that near-occlusion stenosis is not a “surgical emergency,”3,5,9-10 as some surgeons have practiced. In fact, the presence of near-occlusion reduces the stroke risk in comparison to patients with severe stenosis and normal caliber distal ICAs, and thus the benefit of treatment is less.3,5,9-10 A failure to properly identify near-occlusion stenosis puts the patient at risk for misdiagnosis by underestimating the stenosis (when calculating NASCET-style ratios) and at risk for potential inappropriate management decisions.
Near-occlusion is a subset of very severe carotid stenosis. Recognition of near-occlusion has traditionally been a primary interpretive designation with few objective criteria apart from the obvious threadlike “string sign” collapse in the most severe cases.
A recent review of the outcome results of severe stenosis cases with near-occlusion has confirmed prior research, showing a lower stroke risk than in cases without near-occlusion and less benefit from revascularization.5 This review provided helpful diagnostic criteria defining near-occlusion with a greater degree of objectivity. The clinical differences concerning near-occlusion stenosis (less stroke risk and decreased benefit from endarterectomy) warrants attention to methods aimed at recognizing subtle changes of the distal ICA associated with severe carotid stenosis. Our purpose was to increase the objectivity of near-occlusion identification by the addition of numerical data to complement the diagnostic criteria we adapted to CTA from conventional angiography.5 The challenge of identifying near-occlusion with CTA is different from that for conventional angiography, because current CTA techniques are unable to provide the easy ability of sequential imaging during contrast injection. Such temporal imaging provides criteria to determine delayed arrival of contrast and for the presence of collateral vessels.5
Near-Occlusion Diagnosis by CTA
CTA is a relatively noninvasive and quick technique to image carotid arteries as well as intracerebral vasculature. Modern multidetector CTA produces images with high spatial resolution and anatomic detail of not only the contrast-filled lumen, but also the vessel wall and the surrounding soft tissues.11 Multiple studies have verified the ability of CTA to provide an accurate representation of the carotid vasculature in comparison to anatomic phantoms as well as other forms of angiography, including MR and conventional angiography.12-17
The low interobserver variability of all our ICA and ECA measurements supports the high-quality of CTA images, allowing for comparable measurements between independent reviewers by using defined criteria. The greatest measurement variability between reviewers was in the distal ECA, with a correlation coefficient of 0.71 (n = 234). This is not surprising, in light of the greater diameter variability of the distal ECA from its origin at the carotid bifurcation to its final bifurcation into its terminal vessels.
Prior studies have shown that CTA has an excellent correlation with conventional angiography in diagnosing near-occlusion from total occlusion.12 The near-occlusion cases needing distinction from complete occlusion are just the very severe types, with diminutive distal ICAs often referred to as the “string sign” in angiography. The more problematic distinction is between severe stenosis with normal distal ICAs and the more subtle forms of near-occlusion stenosis, with more subtle distal ICA diameter reduction.3,5-10 Because the risk of stroke is less for near-occlusion stenosis and the benefit of revascularization is decreased, proper identification of these cases is necessary for optimal management decisions. Our methods prove that CTA can reliably demonstrate subtle cases of near-occlusion stenosis. Attention to detail, as well as an awareness of this quite distinctive severe stenosis subset, is required for neuroradiologists to identify these subtle changes.
It is not surprising that the best models to detect near-occlusion (dICA: dICA* and dICA models) concern measurements of the distal ICAs, because near-occlusion is defined as severe carotid bulb stenosis causing critical reduction in flow beyond the stenosis, leading to the caliber reduction of the distal ICA. Comparison of the distal ICA with the contralateral distal ICA has a high sensitivity of 97.3% and a negative predictive value (NPV) of 99.4%, an excellent test to rule out near-occlusion.
Nonetheless, the models presented in this report may not be valid for all cases. Even the best model to detect near-occlusion must consider other factors that may influence the diagnosis. For example, near-occlusion does not occur without significant carotid bulb stenosis, so comparison of distal ICAs has no value without severe stenosis. In addition, anatomic variation can occur between ICAs, requiring the reviewer to consider other anatomic clues to explain the differences, such as a congenitally small ICA associated with relatively large anastomoses from the anterior and/or posterior communicating arteries. The most important caution is the distal ICA ratio model (dICA: dICA*), whose calculations are not valid in the presence of contralateral distal ICA disease.
The use of the ipsilateral distal ICA measurement model is simpler than the distal ICA ratio calculations, with nearly similar accuracy (area under the ROC curve = 0.975 [95% CI = 0.952–0.997], compared with that of dICA:dICA* at 0.986 [95% CI = 0.974–0.999]). With a sensitivity of 95.2% and a NPV of 98.9, measurement of the distal ICA is also a good model to rule out near-occlusion. Of course, this model is only valid when associated with a severe carotid bulb stenosis.
The model with the most robust validity is the combination of the 2 top-performing models, the distal ICA ratio and the ipsilateral distal ICA measurement (dICA:dICA* + dICA). This combination improves the specificity of the test to 96.0% and the positive predictive value of 81.0%. The sensitivity of this model is less than either of the 2 models independently, however, remains respectable, at 91.9%. The NPV remains high at 98.4%, again proving to be a good test to rule out near-occlusion.
Conclusion
Most near-occlusion cases have more subtle findings than the less prevalent but better known threadlike “string sign” of severe distal ICA collapse. Detection of near-occlusion is essential to proper diagnosis and treatment of this subset of severe carotid stenosis, because the risk of stroke and the benefit of revascularization is less in these patients. This is especially true because the subtler near-occlusions are less likely to be recognized and are more likely to be managed inappropriately with revascularization as if they were of higher stroke risk with potentially higher treatment benefit as is the case with severe stenosis without near-occlusion.
Detection of these more subtle cases by CTA requires attention to the following criteria: (1) notable stenosis of the carotid bulb and (2) the presence of distal ICA caliber reduction in comparison to (A) its expected size, (B) the contralateral ICA, and (C) the ipsilateral ECA. Threshold values based on these criteria provide guidelines to the CTA interpreter when assessing for carotid artery disease and for the presence of near-occlusion. Ultimately, identification of near-occlusion distal ICAs requires the knowledge of potential reduction in the distal ICA diameter in cases of severe stenosis, a focused search for the subtle near-occlusion characteristics, and the judgment of the interpreter.
References
- Received June 11, 2005.
- Accepted after revision August 10, 2005.
- Copyright © American Society of Neuroradiology