Abstract
BACKGROUND AND PURPOSE: Nimodipine is a therapy that reduces morbidity and mortality in patients with subarachnoid hemorrhage (SAH), though the mechanisms by which it does so are not well understood. In a rabbit model of SAH, we studied the effects of nimodipine by using functional CT imaging. We hypothesized that the nimodipine treatment group would have (1) increased mean basilar artery diameter, (2) less diminished cerebral blood flow (CBF) following vasospasm, and (3) better neurologic outcomes.
METHODS: SAH was induced in 26 New Zealand White rabbits randomized to 2 groups: treated (nimodipine) or control (no treatment). CT perfusion and CT angiography were used to measure CBF and basilar artery diameter at baseline, 10, 30, and 60 minutes after SAH, and on days 3, 5, 7, 9, and 16. Neurologic assessments were performed on each day of scanning.
RESULTS: Basilar artery diameter in the treated group was greater than in the control group post-SAH (P < .05). When vasospasm was >15%, CBF in the nimodipine group was significantly greater than in the control group in the brain stem, cerebellum, parieto-occipital cerebrum, and deep gray matter (P < .05). Neurologic scores in the nimodipine group were significantly better than in the control group on days 5 and 9 (P < .05).
CONCLUSION: Animals treated with nimodipine showed (1) increased mean basilar artery diameter, (2) improved neurologic outcome, and (3) increased mean CBF despite no significant difference in the incidence and severity of delayed vasospasm. These data provide a basis for future studies comparing the efficacy of new treatments for SAH to that of nimodipine.
Subarachnoid hemorrhage (SAH) due to aneurysm rupture is a debilitating condition with serious sequelae. Approximately 70% of patients with SAH will die or remain severely disabled.1 Currently, there are few effective therapies available to improve the poor clinical outcomes associated with SAH.
Delayed ischemic deficits (DIDs) arising within the first 2 weeks of hemorrhage contribute significantly to the high rate of morbidity and mortality associated with SAH.2 Vasospasm and subsequent cerebral ischemia have been identified as major causes of DIDs and deteriorating neurologic status during this time.3–6
In many institutions, nimodipine is used to help prevent ischemic deficits in patients with SAH.7 Nimodipine is a calcium-channel blocker that, along with medically induced hypertension, hemodilution, and hypervolemia, has been shown to improve outcomes in SAH patients significantly.8–10 Despite these encouraging results, in some patients with SAH, nimodipine has been ineffective in preventing or minimizing DIDs.9,11
Inconsistencies in clinical outcome following nimodipine treatment may be because the mechanisms by which nimodipine produces its clinical benefits have yet to be identified. Major hypotheses suggest that nimodipine increases cerebral blood flow (CBF), functions as a neuroprotective agent, or works as some combination of both.12,13 It has also been suggested that nimodipine may increase the diameter of large cerebral vessels affected by vasospasm.8 Nimodipine, however, might not act through a single mechanism; rather, it may produce a clinical effect through a comprehensive relationship between several variables. In this regard, further studies of nimodipine use in SAH are needed to optimize care and to elucidate therapeutic pathways likely to yield improved patient outcomes.14
As new therapies become available for the treatment of DIDs associated with SAH, it will be necessary to develop a method to assess the need for, and the response to, therapy. Recent developments in CT technology offer investigators access to a rapid method of measuring both vessel diameter and tissue blood flow. CT perfusion (CTP) imaging is a widely available and simple way to measure CBF in patients with SAH.15 Likewise, CT angiography (CTA) has been used to identify cerebral vasospasm subsequent to SAH.16 CTP and CTA have the potential to identify the effects of new treatments for SAH when used in combination with, or when compared with nimodipine.
The purpose of this study was to determine the effects of nimodipine on vessel diameter, CBF, and neurologic function in a rabbit model of SAH-related vasospasm. We hypothesized that the nimodipine-treated group would have (1) greater basilar artery diameter than the control group, (2) less-diminished CBF than the control group, and (3) better neurologic outcome than the control group.
Methods
Induction of Experimental SAH
Ethical approval was obtained from the (Canadian) Council on Animal Care and the Animal Use Subcommittee at the University of Western Ontario. Anesthesia was induced in specific pathogen–free New Zealand White (NZW) rabbits (weight range, 2.5–3.5 kg; mean weight, 3.04 ± 0.36 kg) by using 4% isoflurane; an intravenous injection of ketamine/diazepam (valium) mixture (3 mg/kg and 0.3 mg/kg, respectively) was used for intubation. Animals were mechanically ventilated and maintained on 2% isoflurane with vecuronium bromide (0.15 mg/kg). End-tidal CO2 was maintained between 35 and 45 mm Hg and temperature was maintained between 38° and 40°C with a circulating water blanket. Mean arterial pressure (MAP), arterial partial pressure of carbon dioxide (pCO2), arterial partial pressure of oxygen (pO2), and pH were continuously monitored throughout the procedure.
Animals were placed in a prone position on a custom device with their heads secured at a downward angle of 30°. SAH was induced by injecting a single bolus of autologous arterial blood into the cisterna magna.17 In brief, the occipital protuberance was used as a palpable landmark to identify the injection site. By using an aseptic technique, a 23-gauge needle attached to a butterfly catheter was inserted through the skin inferior to the occipital protuberance. When the needle passed into the cisterna magna, slight negative pressure was applied with a 3-mL syringe until clear CSF was observed in the catheter line. As much as 1.5 mL of CSF was withdrawn into the syringe to minimize the effect of volume change during subsequent injection of autologous blood. With the needle still in place, the syringe containing the CSF was replaced with another syringe containing autologous arterial blood taken from the central ear artery. During a 3–5-minute interval, 1.5 mL/kg of blood was injected into the subarachnoid space. The 30° downward tilt of the head, because of gravity, facilitated the formation of a clot around the basilar artery. Unenhanced axial CT images collected immediately following induction of the SAH were used to verify the presence of hyperattenuated blood in the basilar cistern.
Nimodipine Administration
Animals were randomized to a control group (12) or nimodipine-treated group (14). All animals in the treatment group received 2.5 mg/kg of nimodipine (Nimotop) by subcutaneous injection. The first dose was given 1 hour after successful induction of SAH, and thereafter every 24 hours for the duration of the study, to keep plasma concentrations of nimodipine about the target therapeutic level of 7 ng/mL.18 The concentration of nimodipine in the plasma samples was determined with high performance liquid chromatography (HPLC) analysis.18
CT Imaging
All imaging was performed by using a LightSpeed Plus 4 section clinical CT scanner (General Electric HealthCare). Baseline CTA and CTP scanning were performed before SAH induction on day 0. Acute vasospasm (day 0, following SAH) was assessed with CTA scans at 10, 30, and 60 minutes subsequent to the induction of SAH. Development of acute vasospasm confirmed adequate clot formation, thereby ensuring that animals in both treated and untreated groups experienced a similar degree of acute injury. One CTP scan was performed 45 minutes following SAH to determine the acute CBF response.
Delayed vasospasm was assessed with a CTA and CTP scan on days 3, 5, 7, 9, and 16 after induction of SAH. A scout image was used to verify the general position of the head, and axial images were collected to determine the precise locations for CTA and CTP imaging. Nonionic iodinated contrast agent (Omnipaque, 300 mg/mL) was used for both CTA and CTP imaging.
CTA
Two consecutive helical scans through the posterior fossa were performed. The first set of images collected were noncontrast enhanced (NECT). A second, contrast-enhanced scan (ECT) was performed to acquire images at the same section locations as the nonenhanced images while contrast was injected through a catheter in the femoral vein (0.5 mL/s). Section locations were chosen such that the first section was inferior to the vertebrobasilar junction and the last section was superior to the terminal bifurcation of the basilar artery into the posterior cerebral arteries. Image acquisition parameters were as follows: 120 kVp and 60 mA; 512 × 512 image matrix; 12-cm field of view. Because the couch moved through the gantry at a rate of 3.75 mm/s, approximately 70 images were collected with a section thickness of 2.5 mm. Images were overlapped by 2 mm to give a resultant image interval of 0.5 mm in the z axis.
CTP
A 2-cm slab of tissue from the level of the pons to the parieto-occipital cerebrum that included the brain stem, cerebellum, deep gray matter, and parieto-occipital cerebrum was selected and scanned. A total of 99 images were collected during a 25.5-second period at each of 4 5-mm section locations within the slab while the couch remained stationary. The images were acquired at 120 kVp and 60 mA, 512 × 512 image matrix, 12-cm field of view, 1 second per rotation, and a section thickness of 5 mm. Contrast was injected at a rate of 1 mL/s through the femoral vein catheter at the beginning of the 25.5 seconds scan duration.
Neurologic Scoring
A single trained observer blinded to the study design performed all neurologic assessments. The initial assessment was completed between 6 and 12 hours after the SAH (day 0); subsequent assessments were completed on the following days: days 2–3; days 4–5; day 7; day 9; and day 16. For assessments obtained on scanning days, scoring was performed either before or at least 6 hours after anesthesia. The neurologic scale used for the assessments was based on previous work with NZW rabbits.19,20 In brief, clinical observations (spontaneous behavior, reaction to handling, posture, gait, limb hypertonia, righting reflexes, and feeding behavior) were each given a score: 0 (absent); 1 (mild); 2 (moderate); or 3 (severely impaired). Similarly, front and back reflexes were scored: 0 (normal); 1 (brisk); 2 (spreading); or 3 (clonus). Nystagmus was also observed: 0 (absent) or 1 (present). An overall score was calculated as the sum of the individual observations; a greater score denotes more significant neurologic impairment, and a lower score denotes a lesser degree of neurologic impairment.
Data Analysis
CTA.
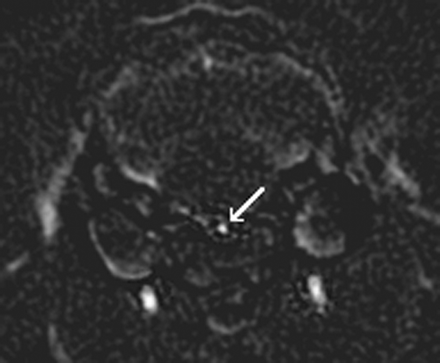
ECT images were subtracted from NECT images to create a set of digitally subtracted CTA images. In the subtracted CT angiographic images, any structures common to both the ECT and NECT images, such as bone and soft tissue, were eliminated, thus allowing the contrast enhanced basilar artery to be clearly identified (Fig 1). Adequate coverage of the basilar artery was ensured by choosing 3 individual segments for analysis: proximal, middle, and distal. Each segment contained 5 cross-sections. By using software developed in our lab (IDL version 5.6, Research Systems Inc.), vertical and horizontal measurements were obtained from each of the 5 sections within each segment.
Subtracted CT angiographic image of a contrast enhanced section through the middle segment of the basilar artery (white arrow).
Horizontal (right-left direction) and vertical (anteroposterior direction) measurements were then averaged together to obtain a single measurement of basilar artery diameter for each of the 5 sections contained within the selected proximal, middle, and distal artery segments. To determine the inner diameter of the basilar artery at each location, measurement units were converted from pixels to millimeters by using an equation derived from phantom experiments previously carried out in our lab (authors’ unpublished data). For each individual segment, measurements from the 5 sections were averaged to provide one distal, one middle, and one proximal measurement of the inner diameter of the basilar artery.
The segment (proximal, middle, or distal) showing the greatest degree of arterial narrowing was identified on the basilar artery to ensure that focal vasospasm (arterial narrowing in only one segment) was measured as well as diffuse vasospasm (arterial narrowing in more than one segment). For each animal, we defined maximum acute vasospasm as the greatest degree of basilar artery narrowing occurring after SAH on day 0. Similarly, we defined maximum delayed vasospasm as the greatest degree of basilar artery narrowing in any one of the 3 segments occurring on post-SAH day 3, 5, 7, 9, or 16. For consistency, the segment (proximal, middle, or distal) in which the greatest degree of maximum delayed vasospasm occurred provided the measurements at all other time points for that individual animal.
Degree of vasospasm was defined as the percent decrease in basilar artery diameter from baseline diameter. We defined 3 categories of vasospasm severity: (1) minimal (vasospasm <15%); (2) moderate (vasospasm 15%–30%); and (3) severe (vasospasm >30%).
CTP.
CBF functional maps were calculated by using CTP software (version 2.6.2, GEHC). Arterial concentration curves were obtained from a 2 × 2 pixel region of interest positioned centrally within the internal carotid artery (ICA). Of the 4 sections in each cine scan, the section containing the ICA with the earliest arrival of contrast was used as the arterial input. To correct for partial volume averaging, a 2 × 2 pixel region of interest was placed on the vessel with the greatest concentration of contrast (the greatest difference between peak attenuation and baseline attenuation on the vessel time-attenuation curve), which was, in all cases, either the superior sagittal sinus or the ICA. A deconvolution between the arterial input curve and the tissue curve provided the brain tissue CBF in 2 × 2 pixel square regions of interest.
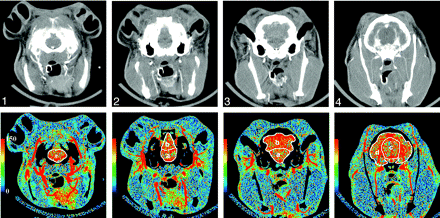
By using software developed in our laboratory (IDL version 5.6), regions of interest were hand drawn around the brain regions (brain stem; cerebellum; deep gray matter; parieto-occipital cerebrum) on functional maps of cerebral blood volume (CBV), which were calculated at the same time as the CBF maps. The regions of interest were automatically positioned onto the corresponding blow flow maps to obtain measurements of CBF (Fig 2). This sequence of drawing regions of interest on CBV maps before positioning them on CBF maps was chosen to limit the effects of large blood vessels on CBF measurements by applying vascular pixel elimination to first exclude regions with CBV > 8.0 mL/100 g and then regions with CBF > 250 mL/100 g/min.21
Top row, Images from a coronally oriented cine CT scan of the rabbit brain from the level of the pons (section 1) to the parieto-occipital cerebrum (section 4). Bottom row, CTP CBF maps corresponding to the CT images. Regions of interest (white) outline the regions in which CBF is quantified in ml/100 g/min. A, brain stem; B, cerebellum; C, deep gray matter; D, parieto-occipital cerebrum.
CBF was measured in each region at each time point. Mean CBF values at each time were determined by calculating the average of the individual CBF measurements made in each region.
Statistical Analysis
Statistical operations were performed by using the SPSS Statistics software package for Windows (version 12.0.1; SPSS Inc., Chicago, Ill). Repeated-measures analysis of variance was used as an omnibus test to identify significant main effects and interactions between the treatment and control group for data collected with CTP and CTA, as well as arterial blood gas parameters and neurologic scores. For the post hoc analyses of the effect of time, paired t tests were used in both the treated and control group. Independent t tests were used to verify differences in parameters between animals in the control group and animals that received nimodipine. Differences in the severity and incidence of maximum vasospasm, as well as mortality rate, were analyzed by using the Fisher exact test. Differences were considered significant at P < .05.
Results
Mortality
Five of 12 (42%) of animals in the control group died or were euthanized within 48 hours of SAH induction compared with 6/14 (43%) of animals in the nimodipine group; this difference was not significant (P > .05, Fisher exact test). The remaining animals in both groups (8 in the nimodipine group and 7 in the control group) survived the duration of the 16-day experiment.
Arterial Blood Gas
There were no differences between the nimodipine and control groups in mean arterial pH, pCO2 or pO2 at any point in time during or following induction of SAH. At 10, 30, and 60 minutes after SAH, arterial pH decreased significantly from baseline in both the nimodipine and control groups (P < .05), though arterial pH remained within normal range (7.35–7.45).
Plasma Nimodipine Concentration
In 5 of the nimodipine-treated animals, blood samples were taken throughout the study to measure nimodipine concentrations in the blood. On the day of sampling, blood samples (1.5 mL) were drawn 22.3 ± 3.5 hours after the previous nimodipine dose was given to measure trough concentrations. The mean trough concentration of nimodipine was 10.6 ± 4.2 ng/mL.
Neurologic Assessments
Average neurologic scores for each group are shown in the Table. Repeated measures analysis indicated significant effects of time (P < .05) and treatment (P < .05) on neurologic score. The neurologic scores obtained on day 0, 6–12 hours after the SAH procedure and recovery from anesthesia, were not significantly different between the control and nimodipine-treated group (P < .05). In the nimodipine group, scores were significantly lower than day 0 scores on each follow-up day (P < .05). Scores for the nimodipine group were significantly lower (less neurologic deficit) than in the control group on day 5 and day 9 (P < .05).
Neurological scores
CTA
Acute Vasospasm.
The treated and control group exhibited similar changes in basilar artery diameter in the acute stage of SAH, before nimodipine treatment; there were no significant differences in basilar artery diameter between the 2 groups at baseline, 10, 30, or 60 minutes after SAH (P > .05). The severity of acute vasospasm seen within 1 hour of SAH induction was similar between the control group and the nimodipine group before treatment. In the nimodipine group, maximum acute vasospasm was 28 ± 18.1%, which was not significantly different from that of the control group, where maximum acute vasospasm was 33 ± 22.4% (P > .05).
Basilar artery diameter was significantly less than baseline measurements in both groups at 10, 30, and 60 minutes (P < .05). There was no significant correlation between the severity of acute vasospasm and mortality within each group (P > .05).
Delayed Vasospasm.
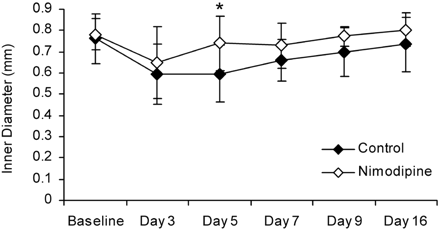
Overall, there was a significant difference in basilar artery diameter between animals in the nimodipine group and control animals (P < .05). Specifically, basilar artery diameter in the nimodipine group was significantly greater than in the control group on day 5 (P < .05; Fig 3).
Basilar artery diameter measured with CTA in the delayed stage of SAH. Error bars represent ± 1 SD. Asterisk indicates significant difference between basilar artery diameter in the control and nimodipine group (P < .05).
There was a significant overall effect of time in both the nimodipine and the control group. Basilar artery diameter was significantly smaller than baseline on days 3, 5, and 7 in the control group (P < .05). In the nimodipine treated group, basilar artery diameter was significantly smaller than baseline on day 3 only (P < .05).
Maximum delayed vasospasm in the nimodipine group (20 ± 6.6%) was less than in the control group (28 ± 16.6%), though this difference was not statistically significant (P < .05). In the nimodipine group, 5/8 animals had moderate vasospasm and 3/8 had minimal vasospasm. In the control group, 2/7 had severe vasospasm, 2/7 had moderate vasospasm, and 3/7 had minimal vasospasm. The incidence of animals that experienced either moderate or severe delayed vasospasm was not different between groups (P > .05, Fisher exact test).
CTP.
Time and region were identified as significant within-subjects factors in the repeated measures analysis (P < .05). Mean baseline CBF values did not differ in any region between the nimodipine and control groups: 63 ± 10.4 mL/min/100 g versus 61 ± 12.2 mL/min/100 g respectively (P > .05). In all regions (brain stem, cerebellum, deep gray matter, and parieto-occipital cerebrum), there was a significant decrease in CBF 45 minutes after SAH in both groups (P < .05). The mean CBF value for all regions at 45 minutes was significantly lower than baseline values in both the nimodipine (52 ± 11.3 mL/100 g/min) and control (44 ± 11.0 mL/100 g/min) groups (P < .05).
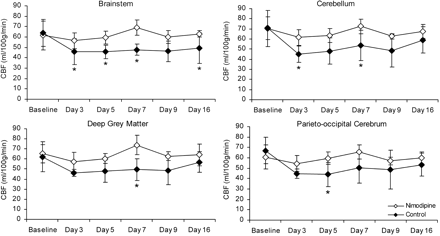
In animals with either moderate or severe maximum delayed vasospasm, mean CBF values were significantly higher in the nimodipine-treated group than in the control group (P < .05; Fig 4). In particular, mean CBF values were greater in the nimodipine-treated group in the brain stem (days 3, 5, 7, and 16), cerebellum (days 3 and 7), parieto-occipital cerebrum (day 5), and deep gray matter (day 7) (P < .05). In animals with either minimal vasospasm or no vasospasm, there was no difference in mean CBF value between treated and untreated groups (P > .05).
Average CTP measurements of CBF (mL/100 g/min) after SAH in the control and treated group. Error bars represent ± 1 SD. Asterisk indicates significant difference between treated and control group (P < .05).
Discussion
In this rabbit model of SAH, we observed acute and delayed vasospasm and changes in CBF by using CTA and CTP imaging. Furthermore, with this CT imaging protocol, we were able to quantify the effect of nimodipine on both delayed vasospasm and CBF after SAH. Our results showed that nimodipine treatment shortened the duration of delayed basilar artery vasospasm, increased CBF in animals with delayed angiographic vasospasm, and improved neurologic condition, validating our 3 study hypotheses.
Cerebral vasospasm and ischemia are 2 important interrelated factors that lead to the development of DIDs in SAH patients.2 The time course and pathophysiology of vasospasm have been extensively studied in animal models and clinical studies.6,22–25 Delayed angiographic vasospasm occurs in approximately 70% of patients with SAH and reaches peak severity approximately 4–10 days after SAH26; evidence of delayed vasospasm has also been shown in several animal models of SAH.17,27–29 Acute and delayed reductions in CBF have been shown to occur in patients with SAH; however, patterns of CBF changes after SAH appear to be more heterogeneous and do not necessarily correlate with vasospasm or neurologic outcome in patients.30,31 Results from previous studies of human subjects show that CBF may decrease,26 exhibit no change,32 or increase because of a hyperemic response after SAH.30 In addition, there are conflicting reports of the correlation between angiographic vasospasm and neurologic outcome.8,33,34 Still, the presence and severity of cerebral vasospasm and ischemia remain important prognostic factors that influence treatment decisions after SAH.35
Nimodipine therapy is part of the standard treatment for SAH patients in most centers.7 The rationale for use of nimodipine as a treatment for SAH-related vasospasm was based on the hypothesis that it would block calcium channels in arterial smooth muscle, thereby reducing the incidence and severity of arterial narrowing and the resulting DIDs subsequent to SAH.36 Despite this rationale, evidence from studies in animals and humans does not consistently show that angiographic vasospasm is reduced with nimodipine treatment. Notwithstanding the lack of clear effect on angiographic vasospasm, nimodipine does reduce neurologic deficits and improve clinical outcomes in patients with SAH.14
Understanding how nimodipine affects the relationship between angiographic (large vessel) vasospasm, cerebral ischemia, and clinical outcome after SAH could aid in the optimization of nimodipine treatment regimens and suggest synergistic therapies to improve outcomes in SAH patients. Furthermore, as new therapies become available, it is likely that study designs will include patients who receive nimodipine together with newer therapies. Thus, more detailed knowledge of nimodipine’s effects on vessel diameter and CBF are becoming increasingly important.37
In response to this need, we have shown that CTP and CTA are feasible methods of quantifying vasospasm and CBF in a rabbit model of SAH-related vasospasm. CTP and CTA imaging are advantageous for repeated study of the angiographic and hemodynamic effects of delayed vasospasm because they do not require sacrifice of the animal to obtain data, allowing for measurements of artery diameter and CBF over the course of 2 weeks in individual rabbits. This strategy is both humane and economical, because the high statistical power of a repeated measures design minimizes the necessary number of animals.
Consistent with previous reports, our model showed the development of acute vasospasm within 10 minutes of SAH induction as well as a corresponding acute reduction in CBF.38 Both groups experienced similar degrees of acute basilar artery narrowing, CBF reduction, and acidosis after SAH induction and before nimodipine treatment. This is important because it means that subsequent differences measured between groups were not likely due to a difference in initial SAH severity between the treated and the control group.
We examined the effect of nimodipine on both basilar artery diameter over the course of post SAH vasospasm and maximum severity of delayed vasospasm (at the time of maximum arterial narrowing). Overall, nimodipine reduced vasospasm after SAH. The repeated measures analysis showed a statistically significant decrease in basilar artery diameter in the untreated group compared with the nimodipine-treated group (Fig 3). Thus, one effect of nimodipine was to decrease basilar artery narrowing post-SAH.
The effect of nimodipine on maximal severity of vasospasm was less conclusive. Although a smaller mean maximal reduction was measured in the nimodipine group (20% vs 28% in the untreated group) this difference did not reach statistical significance. Additional study will be necessary to determine whether nimodipine reduces maximal vasospasm severity in this model.
Our data suggested an effect of nimodipine on the time course of delayed vasospasm in this model. Specifically, nimodipine appears to shorten the duration of time that significant vasospasm is present. Basilar artery diameter in control animals remained significantly lower than baseline until 9 days after SAH; in contrast, basilar artery diameter in the nimodipine group was significantly lower than baseline on day 3 only. This suggests that nimodipine treatment decreased the duration of delayed vasospasm by promoting a faster recovery of the basilar artery to near-normal diameter.
Oxyhemoglobin, a potent vasoconstrictor, peaks in the CSF within 3 days of SAH and begins to decrease before maximum delayed vasospasm.4,39,40 The time course of oxyhemoglobin concentration may be relevant to understanding why significant decreases in basilar artery diameter were evident in the nimodipine-treated group 3 days after SAH but not on later days.
We found that nimodipine had a beneficial effect on CBF after SAH in animals with moderate or severe vasospasm. In a previously published study of normal NZW rabbits, Haws et al showed that there was no significant increase in CBF 30 minutes after an intravenous infusion of nimodipine.41 Our finding of no significant differences in CBF between treated and control animals with minimal vasospasm is consistent with the findings of Haws et al. Nimodipine does not augment CBF when there are minor or no changes in the cerebrovasculature; however, it enhances CBF in animals with more severe vasospasm, likely through the dilation of cerebral arterioles. It would follow then, that in the absence of angiographic vasospasm, autoregulatory function in distal arterioles remains intact negating any potential effects of nimodipine. Our data also support this notion, as a significant effect of nimodipine was apparent only after stratifying the animals into groups based on the presence of moderate or severe delayed vasospasm.
Nimodipine treatment reduced neurologic impairment in this study. Few previously published animal studies have used neurologic scoring to study neurologic deficits after SAH, with or without nimodipine treatment.27,42 We advocate using neurologic scoring as a measure that has greater potential clinical relevance than solely angiographic measurements.
With functional CT imaging, one can acquire in vivo data that are relevant to the clinical problem of SAH. CTP is a widely available method that provides quantitative information about cerebral hemodynamics. It has been used to measure CBF in both SAH and acute stroke patients.15,35,43–45 Clinical studies have shown that CTP can accurately identify ischemic regions in the brain after stroke with a specificity of 93%.44 CTA is gaining acceptance as a standard method of diagnosing vasospasm and aneurysm location in SAH patients. The ability of CTA to identify vasospasm appears to be similar to that of the “gold standard” test, digital subtraction angiography (DSA).16,46 In addition, studies have indicated that CTA can detect aneurysms with a sensitivity of 100%.47,48 Compared to DSA, CTA is noninvasive and carries lower risk.
The functional imaging protocol we used in this study differs somewhat from CTA and CTP scanning protocols in humans. The duration of CTP scanning in human patients is longer (2 minutes) than in our animal protocol (25.5 seconds).15 This discrepancy is likely due to the longer circulation time in humans compared with rabbits. This difference in circulation time also explains why CTA in humans requires a 25-second delay between contrast injection and data acquisition, whereas only an 8-second delay was necessary for CTA in rabbits.48
CTA images in this study were processed by subtracting contrast-enhanced images from unenhanced images. The subtraction method, in conjunction with the use of anesthesia and muscle paralytics during artificial ventilation, helped to improve the signal-intensity-to-noise ratio of the resultant angiogram images, thereby increasing the accuracy of quantitative basilar artery measurements. In contrast, data collected with human CTA would not require subtraction because1 the larger vessel size makes it unnecessary for a qualitative assessment of vessel stenosis, and2 patient respiratory motion is greater, making subtraction a less-effective strategy for improving the signal intensity to noise ratio of resultant angiogram images.
There are some practical limitations to the techniques used in this study. First, although most of the blood pooled around the basilar artery in the posterior fossa, as seen on unenhanced CT immediately following SAH induction, we could not fully control for the final location of subarachnoid clot. Second, neurologic assessments are difficult to perform in rabbits. Although our neurologic evaluations were completed by a trained observer blinded to treatment status, there was some degree of subjectivity to the scoring scheme. Third, our repeated measures study design, while efficient for studying vasospasm, made monitoring of intracranial pressure during CBF measurements impractical. In conjunction with MAP, intracranial pressure has a direct effect on cerebral perfusion pressure.
Conclusion
In a rabbit model of SAH-related vasospasm, we showed that nimodipine treatment increased CBF, improved neurologic outcome, and reduced the duration of delayed vasospasm. We also demonstrated the utility of CTP and CTA imaging techniques for evaluating nimodipine’s effect on SAH and assessing the potential of new therapies in the presence of, or in comparison to, nimodipine.
Acknowledgments
We thank Dominique Ouimet and Jennifer Hadway for their assistance with the animals used in this study. We also thank Brad Urquhart and David Freeman (Department of Physiology and Pharmacology, University of Western Ontario) for analyzing the plasma samples collected in this study.
Footnotes
Grant support: American Society of Neuroradiology—Foundation Scholar Award (to J.D.E.) and Canadian Institutes of Health Research, Ontario Research and Development Challenge Fund (BRAIN consortium).
References
- Received May 20, 2005.
- Accepted after revision August 10, 2005.
- Copyright © American Society of Neuroradiology