Abstract
BACKGROUND AND PURPOSE: Human herpesvirus-6 (HHV-6)-associated encephalopathy tends to develop in immunocompromised patients. Neurologic symptoms, such as disorientation, short-term memory loss, convulsion, coma, and hypopnea could occur, but they may be nonspecific. We retrospectively reviewed MR images of 6 adults with HHV-6-associated encephalopathy to study characteristic imaging findings that could be useful in making the diagnosis.
MATERIALS AND METHODS: Between 2003 and 2005, we encountered 6 cases of HHV-6-associated encephalopathy (3 men and 3 women; age range, 36–55 years) in 3 hospitals. The diagnosis was made clinically according to the neurologic symptoms accompanied by high-level copies of HHV-6 DNA in CSF or peripheral blood by quantitative polymerase chain reaction without the detection of any other infectious pathogen.
RESULTS: All 6 patients had abnormal hippocampus/amygdala findings on presentation, and no other regions were involved. In the early period (0–2 days from onset), abnormal high signal intensity on fluid-attenuated inversion recovery (FLAIR) imaging (2 of 3, 67%) and on diffusion-weighted images accompanied by apparent diffusion coefficient (ADC) reduction (2 of 2, 100%) were observed. In the middle period (3–30 days), abnormal low signal intensity on T1-weighted images (5 of 6, 83%) and abnormal high signal intensity on T2-weighted images (4 of 6, 67%) and FLAIR (5 of 6, 83%) were confirmed. In the late period (> 30 days), we saw the resolution of signal intensity abnormalities and the appearance of atrophic change (4 of 4, 100%) of the affected regions.
CONCLUSION: HHV-6-associated encephalopathy in adults tends to affect the mesial temporal lobe. MR imaging is useful for detecting HHV-6 encephalopathy and distinguishing it from the other diseases of the central nervous system in immunocompromised patients.
Human herpesvirus-6 (HHV-6) is a double-stranded DNA virus belonging to the β-herpesvirus subfamily and is also known to be one of the causative pathogens of exanthem subitum. HHV-6 excreted by the salivary glands of a mother is contagious to her child in infancy, and more than 90% of the general population is seropositive for HHV-6.1,2 Recently, HHV-6 has been recognized as a serious pathogen in immunocompromised patients,3 and several case studies of HHV-6-associated encephalopathy have been reported in patients who have undergone hematopoietic stem cell or solid-organ transplant.3–10 Immunocompromised patients may suffer from various disorders of the central nervous system (CNS), but their symptoms may be nonspecific. It is important to clarify the characteristics of the imaging findings of HHV-6-associated encephalopathy to differentiate it from the other diseases. Nevertheless, so far only a few studies have closely evaluated the imaging findings of HHV-6 encephalopathy.4–7 In the present study, we retrospectively analyzed the imaging findings of 6 immunocompromised patients with HHV-6-associated encephalopathy.
Materials and Methods
Between 2003 and 2005, 6 patients (3 men and 3 women; age range, 36–55 years; median age, 45 years) in 3 hospitals were diagnosed with HHV-6-associated encephalopathy (Table 1). It is usually very difficult to make a definitive diagnosis of HHV-6 encephalitis.11 To prove that HHV-6 is the cause of the neurologic symptoms requires positive quantitative polymerase chain reaction (PCR) in the CSF and increase of IgG antibodies against the HHV-6 in paired serum, considering that a high percentage of the population is seropositive for HHV-6.2 In this study, the diagnosis was made clinically, based primarily on the development of neurologic symptoms accompanied by a high level of copies of HHV-6 DNA in CSF (patients 1, 2, 4, 5, and 6; 2300–58,000 copies/mL) or an excessively high level of copies in peripheral blood (patient 3; 400,000 copies/mL) detected by quantitative PCR without the detection of any other infectious pathogen. Findings in head MR images were used as a supplement. Definitive diagnosis based on paired IgG antibodies was not made in any of the 6 patients. Nevertheless, a strong association between the neurologic symptoms and the increase of HHV-6 DNA in the specimen was suspected in each patient.
Summary of patient histories
All CT studies were performed on Aquilion CT scanners (Toshiba Medical Systems, Otawara, Japan) with a section thickness of 2 to 10 mm. MR imaging studies were carried out on Magnetom Vision or Symphony units (Siemens, Erlangen, Germany). With respect to imaging protocols, various imaging techniques were adopted according to the policy of each institution and the conditions of patients. T1-weighted images (T1WIs; TR/TE = 411–624/11–17 ms) and T2-weighted images (T2WIs; TR/TE = 2500–3800/93–99 ms) were performed in all examinations. Fluid-attenuated inversion recovery images (FLAIRs; TR/TE/TI = 8000–9000/91–110/2200–2300 ms), diffusion-weighted images (DWIs; TR/TE = 3100–4000/104–137 ms, maximum b factor = 1000 s/mm2), apparent diffusion coefficient (ADC) map, or contrast-enhanced T1WIs (TR/TE = 525–584/17 ms; 0.1 mmol/kg Gd-DTPA, Magnevist, Nihon Schering, Osaka, Japan) were performed in some examinations.
We reviewed the medical chart of each patient to collect the following clinical data: 1) the interval between transplantation and the onset of neurologic symptoms, 2) neurologic symptoms, and 3) outcome.
Head CT and MR axial images were analyzed by 2 expert neuroradiologists (T.N., T.Y.) in a consensus fashion. For the image analysis, we expediently divided the observation period into 3 periods: 1) the early period, defined as within 2 days of the onset of neurologic symptoms; 2) the middle period, from 3 to 30 days after the onset of symptoms; and 3) the late period, more than 30 days from the onset of symptoms. The early, middle, and late periods were intended to represent 1) early acute, 2) late acute to subacute, and 3) chronic phases of the disease, respectively. When ADC maps were available, ADC changes in the affected region were quantitatively evaluated. Because of the inconsistent DWI imaging parameters (TE and b factor), direct comparison of absolute ADC values was not attempted. Rather, we calculated ADC ratios for each patient by dividing the ADC in the lesion by that in the normal brain parenchyma. The ADC in the lesion was measured as a mean value in an ovoid region of interest placed within the anterior part of the right hippocampus. The ADC of the normal parenchyma was measured in the normal-appearing white matter region in the right temporal lobe.
Results
The interval between transplantation and the onset of neurologic symptoms ranged from 11 to 979 days (median, 20.5 days), and it fell within 4 weeks after transplantation in 5 of our 6 patients. Patient 2 underwent the transplantation about 32 months ago, and there may be no direct association between HHV-6 encephalopathy and transplantation. Instead, she underwent the chemoradiation treatment for 4th recurrence of acute leukemia, and she developed the neurologic symptoms 47 days after the start of the treatment. The other 5 patients had the preventive administration of acyclovir for a month after transplantation. The most common neurologic symptoms were disorientation (4 of 6, 67%) and short-term memory loss (4 of 6, 67%) followed by coma (3 of 6, 50%), hypopnea (3 of 6, 50%), and convulsion (3 of 6, 50%). The survival rate was 50% (3 of 6). Three patients died: one from sepsis caused by Pseudomonas aeruginosa, 1 another from interstitial pneumonitis due to respiratory syncytial virus (RSV), and the last from liver failure due to graft-versus-host disease. Among these 3 patients, autopsy was performed only on patient 5, who died of RSV pneumonitis on day 222 after the onset of the symptoms. Postmortem findings from this patient are described below.
Head CT, which was performed in 3 of the 6 patients during the early period, showed no remarkable lesions. On MR, all 6 patients presented with abnormal findings in the hippocampus and/or amygdala either bilaterally (patient 1–5) or unilaterally (patient 6, right). No abnormality was found in other brain regions in any patient. MR abnormalities during the 3 periods are summarized in Table 2. In the early period, an abnormally high signal intensity was found on FLAIR (2 of 3, 67%) and DWI (2 of 2, 100%); the DWI abnormality was accompanied by ADC reduction in both patients (2 of 2, 100%). In the middle period, there was an abnormally low signal intensity on T1WIs (5 of 6, 83%) and an abnormally high signal intensity on T2WIs (4/6, 67%), FLAIR images (5 of 6, 83%), and DWIs (3/5, 60%). In the late period, the signal intensity abnormalities disappeared, and atrophic change of the affected regions took place (4 of 4, 100%). No abnormal contrast enhancement was detected in any period. MR images of a representative patient are shown in Fig 1. Figure 2 shows sequential changes in the FLAIR images, DWIs, and ADC maps of another patient.
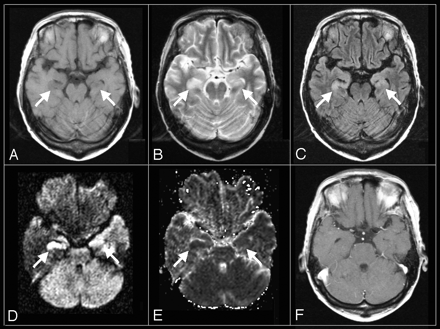
Axial MR images of an HHV-6 encephalopathy patient (patient 2) obtained on the second day after the onset of neurologic symptoms.
A, T1WI (TR/TE = 466/11 ms).
B, T2WI (2650/93 ms).
C, FLAIR (TR/TE/TI = 9000/97/2300 ms).
D, DWI (TR/TE = 3100/119 ms, b = 1000).
E, ADC map.
F, postcontrast T1WI (TR/TE = 558/17 ms).
An abnormal low signal intensity on T1WI (A) and high signal intensity on T2WI (B) and FLAIR (C) are shown in the bilateral amygdalae and hippocampi. High signal intensity on DWI (D) with ADC reduction (E) is also shown. However, no abnormal enhancement is seen on postcontrast T1WI (F).
Serial axial MR images of a 49-year-old woman (patient 1) including FLAIR (TR/TE/TI = 9000/110/2200 ms [A, B, D] or 9000/97/2300 ms [C]), DWI (TR/TE = 4000/137 ms [E, F, H] or 3100/119 ms [G]), and ADC maps on days 1, 13, 26, and 98 after the onset of neurologic symptoms (I-L). On FLAIR, high signal intensity in the bilateral amygdalae and hippocampi appears on day 1, peaks on day 13, and becomes less pronounced on day 26, disappearing on day 98 but leaving marked atrophy. High signal intensity on DWI is observed until day 26, whereas ADC value reduction is seen only on days 1 and 13.
MR findings in the amygdala/hippocampus and the time interval from the onset of neurological symptoms
The ADC ratios are tabulated in Table 3. Maps of ADC were available in 9 examinations of 3 patients, including 2 examinations of 2 patients in the early period, 4 examinations of 3 patients in the middle period, and 3 examinations of 2 patients in the late period. In the early period, moderate to severe ADC reductions (range of ADC ratio: 59%–77%) were observed in both of the patients. In the middle period, divergent results ranging from mild reduction to mild elevation were seen (range of ADC ratio: 83%–114%). In the late period, ADC was abnormally elevated in all 3 examinations (range of ADC ratio: 115%–137%).
ADC ratio of abnormal to normal brain parenchyma
Postmortem examination of brain tissue from patient 5 revealed small necrotic changes in bilateral hippocampi and small hemorrhagic lesions in the cerebrum, midbrain, and pons. He underwent head MR imaging on days 1, 13, 26, and 98 after the onset of neurologic symptoms. On day 1, no abnormal findings were observed on T1WI, T2WI, or FLAIR images. On day 13, abnormally low signal intensities on T1WI and high signal intensities on T2WI, FLAIR, and DWI were seen in the bilateral hippocampi. The abnormal signal intensity became unclear, and an atrophic change was recognized on day 96. The patient underwent the antiviral treatment and recovered, leaving the sequela of short-term memory loss. Unfortunately, he died of interstitial pneumonitis due to RSV on day 222 after the onset of the HHV-6 encephalopathy.
Discussion
HHV-6 has a strong affinity for the CNS and shifts to the CNS at the time of the primary infection at a high rate.12 In a previous report, HHV-6 DNA was detected by PCR in approximately one third of 31 normal brain tissue specimens, suggesting that brain tissue might be a latent viral site.13
HHV-6-associated encephalopathy has been increasingly recognized as a serious complication in immunocompromised patients. There have been many reports of HHV-6 encephalopathy related to transplantation, such as hematopoietic stem cell,3–7 lung,8 and liver9 transplantation, though HHV-6 encephalitis associated with drug sensitivity syndrome10 has also been reported. In most transplantation-related cases, the pathogenesis is considered to be the reactivation of the recipient’s latent HHV-6, but not infection from a donor.14 A pathologic report of HHV-6-associated encephalopathy by Wainwright et al4 found subacute sclerosis in the bilateral hippocampi characterized by severe neuronal loss and reactive astrocytosis with the detection of HHV-6 P41/P101 protein, whereas the bilateral thalami, brain stem, spinal cord, and cerebellum were unaffected. In addition, previous clinical reports with MR imaging findings have shown predominant mesial temporal lobe abnormalities, including abnormal high T2 signal intensities and early volume loss in the hippocampus.4–7 The exclusive involvement of the mesial temporal structures (hippocampus and amygdala) revealed in the present study is consistent with these previous pathologic and clinical imaging reports, though no acceptable mechanism provides for this finding.
To the best of our knowledge, ours is the first report to document sequential changes in the MR images of patients with HHV-6-associated encephalopathy. An abnormally high signal intensity in the hippocampus and amygdala on T2WI, FLAIR, and DWI was the main finding in the early and middle periods, whereas the high signal intensity faded in the late period, leaving focal atrophy. To the best of our knowledge, there has been no previous report on DWI of patients with HHV-6-associated encephalopathy. The abnormal high signal intensity on DWI with ADC reduction in the early period in our 2 patients suggests that the acute pathology of HHV-6-associated encephalopathy includes cytotoxic edema rather than vasogenic edema.15 Similar acute changes in DWIs and/or ADC maps have been reported among patients with encephalopathy due to the influenza virus,16 HHV-6 on primary infection,17 and other viral or bacterial pathogens.18 Our results of ADC ratios showed early ADC reduction followed by abnormal ADC elevation in the late period, which may reflect the aspect of histopathologic transition from early cytotoxic edema to subsequent necrosis. The postmortem findings in the hippocampus of patient 5 appear consistent with this speculation.
Because of small patient numbers and inconsistent imaging protocols, the relative sensitivities among the different imaging methods cannot be determined. However, we believe that FLAIR and DWI may be more sensitive than T2WI in detecting early changes related to HHV-6-associated encephalopathy because the CSF signal intensity is suppressed in FLAIR, and DWI highlights the lesion. Moreover, imaging in the coronal plane may be useful to evaluate the entire hippocampus.
In transplantation, acyclovir is routinely administered to prevent reactivation of herpes viruses. However, acyclovir is not effective against HHV-6 because of its lack of virus-specific thymidine kinase.19 Ganciclovir and foscarnet can be effective against HHV-6, but adverse effects, including serious myelosuppression or nephrotoxicity, are of great concern.8 Therefore, prophylactic use of these drugs is not usually performed. Early diagnosis is critical to prevent serious neurologic sequelae.
Differential diagnosis on MR images may include herpes simplex encephalitis (HSV encephalitis), other viral encephalopathies, the CNS invasion of lymphoproliferative diseases, paraneoplastic encephalopathy, and drug-induced encephalopathy. However, the combination of the characteristic mesial temporal involvement on MR images and the immunocompromised condition (especially from 2 to 4 weeks after transplantation) under the preventive administration of acyclovir is highly suggestive of HHV-6-associated encephalopathy.
The MR appearances of HSV encephalitis and HHV-6-associated encephalopathy seem similar in that they both have hippocampus and amygdala involvement. MR findings of HSV encephalitis have been reported as the multifocal confluent lesions, the leptomeningeal or cortical enhancement on contrast-enhanced T1WI, and occasional cerebral hemorrhage.20,21 With respect to the DWI findings of HSV encephalitis, the affected region showed a mixture pattern of the reduction and elevation of ADC, which may reflect the mixture of cytotoxic edema and vasogenic edema.22 These imaging findings of HSV encephalitis are considered more severe and widespread than those of HHV-6 encephalopathy observed in our cases and other reported cases, although they may be similar in some cases.
We retrospectively analyzed the MR findings of 6 immunocompromised patients with HHV-6-associated encephalopathy. In our series, the mesial temporal lobes were exclusively affected. We have reported sequential changes in the MR findings of our patients. MR may be useful for the detection and follow-up of HHV-6-associated encephalopathy in immunocompromised patients.
References
- Received October 31, 2005.
- Accepted after revision January 25, 2006.
- Copyright © American Society of Neuroradiology