Abstract
SUMMARY: Head and neck surgeons often rely on imaging to determine if a neoplasm is resectable. Many of the critical issues are outlined in the American Joint Committee on Cancer Staging Manual, wherein T4a and T4b head and neck cancers are defined as resectable and unresectable, respectively. Even within the T4a advanced resectable classification, there are critical determinants that define whether the surgical option is such that major morbidity and mortality could be expected. This review article examines the imaging literature to determine the accuracy and diagnostic criteria of different modalities for evaluating these critical T4a and T4b factors, which include the following: 1) arterial encasement, 2) prevertebral fascia involvement, 3) mediastinal infiltration, 4) tracheal and esophageal extension, 5) laryngeal cartilage penetration, 6) pre-epiglottic fat involvement, 7) dural spread, 8) bone (mandible/maxilla and skull base) infiltration, 9) perineural spread, 10) orbital involvement, and 11) brachial plexus invasion. For the most part, the studies find MR imaging with higher sensitivity but lower specificity than CT. An ever-increasing role for PET/CT is suggested. Imaging is of great value in the determination of resectability issues listed previously for head and neck cancers, with the possible exception of prevertebral fascia involvement.
In 2002, the American Joint Commission on Cancer (AJCC) revised the T-staging classifications of head and neck cancers.1 The most advanced staging, the T4 classification, was separated in large part by subsite of the head and neck mucosal system into T4a and T4b designations. The purpose of this separation into T4a and T4b was to emphasize the poor prognosis and high rate of unresectability in T4b classification tumors. Most patients with T4b tumors, therefore, were generally categorized as unresectable, and the suggestion was that they should preferentially be offered medical therapy, be it with chemotherapy, radiation, or a combination thereof. On the other hand, the T4a tumors were also lesions that would require extensive surgery but were still classified as resectable tumors. (Table 1).
T4a versus T4b tumor classifications for various sites of head and neck cancers1
In the evaluation of a patient with head and neck cancer, a conflict exists between what it would take to completely resect an advanced cancer versus the impact such a resection would have on the patient’s quality of life and self-image. Head and neck cancers are analogous to brain tumors; although it may be possible to obtain a gross total resection, the morbidity may create a compromised quality of life of questionable additional value. Many patients have to think long and hard about the prospects of the surgical deformities of the face with respect to head and neck cancer before deciding whether to go with surgical treatment versus combination chemotherapy—radiation therapy (hereafter called “medical therapy”). Would you take the surgical option that requires an orbital exenteration if it only leads to a 10% improvement in 5-year prognosis over another treatment that spares the orbit? Would you be willing to have your entire larynx removed and have to speak via artificial means if hypothetically, the improvement in prognosis over medical therapy is to increase 5-year survival from 20% to 35%? Obviously, these decisions are individual and based on self-image, career issues, age, and future expectations.
For these reasons, the critical issues regarding head and neck cancer resectability, both from the standpoint of long-term prognosis as well as the morbidity of the patient, must be considered closely. The purpose of this review article is to discuss the imaging findings regarding those critical issues that are the basis for the patient designation as 1) unresectable, 2) resectable with limited “collateral damage” and morbidity, or 3) resectable but requiring extensive complicated surgery with these quality-of-life issues.
It is important to understand that unresectability does not necessarily imply incurability. The classic example of this is nasopharyngeal carcinoma, with which one can have extensive disease into the intracranial compartment, yet chemotherapy and radiation therapy may be curative. Additionally, the histology of the tumor may be critical, with some histologies (like adenoid cystic carcinoma) providing long-term survival even in “unresectable” diseases. Whereas the subsequent discussion of the AJCC criteria refers to squamous cell carcinoma (SQCC), it is clear that many patients who have lesions such as lymphoma can have no evidence of residua after therapy, even with initial “unresectable disease” (vascular encasement, prevertebral musculature infiltration, or mediastinal infiltration) before therapy.
T4b Issues
The AJCC classification includes 3 repetitive criteria for the T4b cancers across most aerodigestive system sites (Table 1). These are 1) vascular encasement and invasion, 2) prevertebral space invasion, and 3) invasion of mediastinal structures.
Vascular Encasement
The process of vascular invasion was extensively studied by Niimi et al 2 in 26 surgical and autopsy specimens of SQCC of the oral and maxillofacial regions. They observed mechanical disruption of the vascular walls by carcinoma cell nests, which subsequently invaded the lumen as clusters of cells. At the vascular invasion sites, inflammatory reactions were seen around carcinoma cell nests, inside as well as outside the lumina, with microthrombotic reactions due to endothelial injury.
The diagnostic value of CT in detecting vascular invasion by head and neck malignancies has been assessed by different groups. Yu et al3 described 6 types of vascular involvement of the carotid artery and jugular vein on CT in 43 patients with head and neck malignant tumors. The highest accuracy (84.1%) was recorded in 2 types: compression and deformation of the common carotid artery (CCA) or internal carotid artery (ICA) and partial fat or fascia deletion between tumor and the CCA or ICA. Circumferential vessel-wall involvement of greater than 180° on CT had a sensitivity as low as 18.5%. The authors concluded that the accurate diagnosis of carotid artery involvement by CT was difficult.
Yoo et al4 evaluated 34 patients with head and neck cancer who underwent carotid artery resection based on the clinical impression of tumor invasion. They used a single CT criterion of 180° circumferential tumor attachment. However, they found that clinical assessment was as predictive as CT for tumor invasion. Moreover, Nix and Coatesworth5 compared the CT scans and the operative findings of 196 patients with an upper aerodigestive tract SQCC and indicated that CT significantly overestimated carotid artery invasion.
Ultrasonography is routinely used in the evaluation of the carotid arteries and other superficial structures of the neck because of its noninvasiveness, reliability, and cost-effectiveness. Mann et al6 developed a helpful ultrasonographic staging system (Table 2) for carotid artery involvement based on findings from 41 patients with extensive metastatic neck disease. Five patients with stage III and IV underwent transcranial Doppler (TCD) sonography to determine crossflow in the middle cerebral artery (MCA) with compression testing of the CCA. They concluded that a crossflow circulation of the MCA by using TCD demonstrating greater than 90% of flow velocity obtained under normal conditions within 20 seconds after external compression of the carotid artery allows safe resectability of this vessel.
Sonographic classification of vascular resectability6
Vascular encasement was evaluated by Yousem et al7 with regard to MR imaging findings suggestive of unresectable arterial encasement. This study used MR imaging and looked at the extent of circumferential involvement of the vascular wall, intraluminal tumor, and focal signal-intensity abnormalities in the vascular wall compared with the gold standard of surgical evaluation. The study population included 29 patients with SQCC in which the tumor was believed to be fixed clinically to the CCA or ICA or in close proximity to the carotid or vertebral artery.
The authors found that the single criterion of involvement of 270° or more of the circumference of the carotid artery was accurate in predicting the surgeon’s inability to peel the tumor off the carotid artery in 100% of the cases.7 This included 10 positive cases and 19 negative cases for vascular invasion. For those cases in which the artery could not be salvaged, temporary balloon occlusion was performed with resection of the tumor, and histopathologic confirmation was performed for vascular invasion. In the entire dataset of 49 patients and 53 arteries, there were 6 false-positive cases that were not SQCC, including 4 chordomas. However, for the SQCC, the 270° rule was 100% accurate. Intraluminal tumor, though specific for vascular infiltration and unresectability, was infrequently seen and not very sensitive.
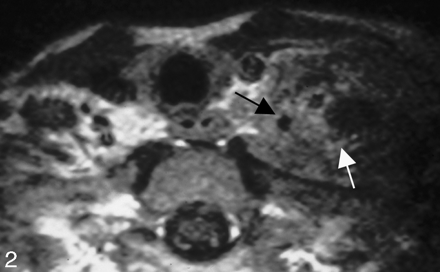
Subsequently numerous articles have been published demonstrating similar findings that the circumferential involvement of the vessel (Figs 1 and 2) is most useful in determining that a tumor is unresectable on the basis of vascular infiltration. Most of these studies include cases of both primary tumor involvement of the encased vessel as well as infiltration from adjacent adenopathy.
Carotid encasement; adenoid cystic carcinoma. A, Note the circumferential involvement of the left cavernous artery by a soft-tissue mass with narrowing of the carotid artery in this patient who had adenoid cystic carcinoma from the sinonasal cavity. B, This patient also demonstrates erosion of the clivus with bony infiltration of the skull base.
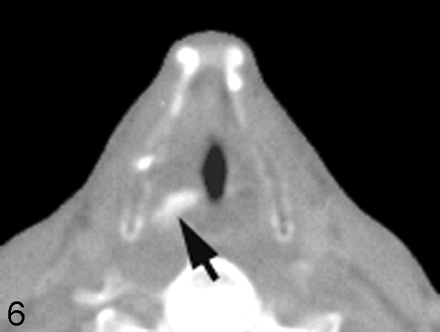
Carotid fixation. This patient who had recurrent thyroid cancer in the left side of the neck demonstrates effacement of the fat planes around the left CCA laterally (black arrow). Nonetheless, the tumor does not extend greater than 270° around the circumference of the carotid artery, and the tumor was able to be stripped off the carotid artery at surgery. Note also the tumor abutting the anterior scalene muscle on the left side (white arrow). In many instances, the head and neck surgeons believe that this is a contraindication for further surgery due to the potential for brachial plexus involvement.
Prevertebral Fascia Involvement
Another critical criterion for the T4b classification is the presence of prevertebral fascia involvement, defined clinically by fixation of the tumor to the prevertebral musculature. Trying to strip tumor from the longus colli/capitis muscle complex has been shown to be fraught with difficultly and does not improve a patient’s long-term prognosis because there is often residual disease. In addition, the presence of prevertebral infiltration is associated with a high rate of retropharyngeal and other site adenopathy, again leading to a worse prognosis. Fixation to the prevertebral musculature is difficult to evaluate clinically because it requires a manual assessment of the mobility of the tumor with the muscular column and vertebral column. The tumors that usually infiltrate the prevertebral space are ones that are arising from the posterior mucosal wall, be it from nasopharynx, oropharynx, hypopharynx or, rarely, extensive laryngeal, thyroid, or esophageal cancers.
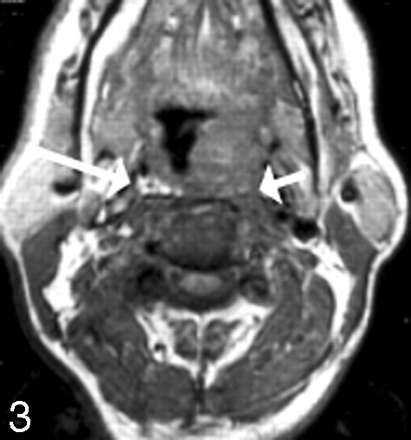
Hsu et al8 demonstrated that the preservation of a retropharyngeal high-signal-intensity fat stripe on sagittal or axial T1-weighted scans served as an excellent indicator that tumors had not become fixed to the prevertebral fascia or musculature. This finding makes sense in that mucosal lesions largely must cross the retropharyngeal space, usually containing fat, lymph nodes, and connective tissue, to broach the longus colli/capitis muscle complex (Fig 3). The width of this fat stripe is variable from patient to patient and from superior to inferior, being more abundant in the superior region. Hsu et al found that 39 of 40 patients with preservation of the retropharyngeal fat stripe on T1-weighted scans showed absence of infiltration of the prevertebral musculature. Thus, this is a relatively specific finding for excluding prevertebral musculature invasion.
Prevertebral muscular infiltration. This T1-weighted scan demonstrates a left tonsillar carcinoma, which causes effacement of the fat of the retropharyngeal space on the left. Compare the left side with the right side. The irregular margin of the muscle belies the infiltration of the longus colli in this patient with fixation of tumor.
Loevner et al9 evaluated the findings that would suggest the presence of prevertebral musculature invasion. These included abnormal longus muscle concavity, abnormal T2 hyperintensity in the prevertebral musculature, abnormal contrast enhancement in the prevertebral musculature, and an irregular border to the tumor’s interface with the prevertebral musculature. The authors found that none of the 4 findings evaluated showed an accuracy rate greater than 60%. Although a criterion might show sensitivity as high as 88% (as in bright T2-weighted signal intensity), that same criterion had specificity as low as 14%. The striking finding of the study, however, occurred in 7 patients who had all 4 abnormalities—loss of the fat stripe, nodularity within the prevertebral musculature, abnormal signal intensity in the prevertebral musculature, and gadolinium enhancement of the prevertebral musculature. Four of the 7 patients did not show prevertebral musculature infiltration when evaluated at surgery.
It is useful to understand the surgeon’s evaluation of the infiltration of the prevertebral fascia in the operating room. In most cases, the surgeon who suspects prevertebral musculature infiltration attempts to assess the fat plane on the side opposite the suspected region of prevertebral invasion. He or she therefore, first creates a plane with dissector and/or finger on the normal contralateral side, hoping to extend that plane of dissection across to the opposite side. If that plane of dissection cannot be created and there is tumor preventing the dissection, then the surgeon will close the wound and refer the patient for medical therapy having deemed the patient unresectable. Loevner at al9 concluded that this surgical or endoscopic assessment (in which the tumor can be palpated and manipulated to assess fixation without surgical dissection) should continue to be the standard of care at this time, with limited assistance by imaging unless there is an intact high-signal-intensity fat stripe on the T1-weighted scans as per Hsu et al.8
Subsequently, other authors have evaluated patients with prevertebral muscular invasion and nasopharyngeal cancer. They have found that in these patients with fascial invasion, in whom surgical treatment is not offered because standard radiation and chemotherapy are so effective and surgery incurs unacceptable morbidity, the prognosis is much worse. The chance of local recurrence and lymphadenopathy is much higher, and the 5-year prognosis is much worse. Even hematogenous metastases are increased in patients with nasopharyngeal carcinoma and prevertebral infiltration.10
Mediastinal Invasion
The third criterion for unresectability in the T4b category is mediastinal invasion. This is obviously much less common in patients with suprahyoid aerodigestive system masses but can be seen on rare occasions in patients with subglottic laryngeal, hypopharyngeal, thyroid, and esophageal cancers. The criteria for mediastinal invasion have not been well studied however, empirically; infiltration of mediastinal fat and vascular invasion of their supra-aortic vessels and/or the aortic arch in a circumferential manner would be indicative of mediastinal invasion. In many instances, head and neck surgeons may define an inferior border below which they feel they can no longer resect tumor without the participation of a thoracic surgeon. This is usually stated in terms of the sternal notch in that tumors below the sternal notch are more difficult to resect via a cervical approach than those above the sternal notch. Mediastinal invasion may include tracheal and esophageal infiltration.
Wang et al11 evaluated the accuracy of 3 MR imaging criteria in the prediction of tracheal invasion in 67 patients with thyroid carcinoma.12 Thirty healthy subjects and 1 cadaver underwent MR imaging of the trachea as a reference standard. Criteria of invasion were 1) soft-tissue signal intensity in the cartilage, 2) intraluminal mass, or 3) circumferential tracheal abutment of 180° or greater. There were 7 false-positive findings using these criteria. The authors indicated that a soft-tissue signal intensity was normally seen in healthy tracheal cartilage and sometimes represented tumor extension between the cartilaginous rings without invasion. The highest accuracy (90%) was achieved when using a combination of any of the 3 findings yielding sensitivity of 100% (23/23) and specificity of 84% (37/44). These findings were associated with a much worse overall prognosis from thyroid cancer.11
Karwowski et al13 demonstrated the use of intraoperative ultrasonography (IOU) in the identification of recurrent thyroid cancer in 13 patients. They inserted the sterile-covered transducer directly into the skin incision. Areas of recurrent tumor were seen as hypoechoic masses with increased color Doppler flow, indicating their vascular nature. IOU was particularly useful in detecting paratracheal tumor or thyroid cartilage invasion by nodules 20 mm or less.
Intraluminal infiltration of the trachea or the esophagus is a late sign of their involvement by neck tumors. King et al14 evaluated MR imaging in the staging of papillary carcinoma of the thyroid with operative findings as a gold standard.15 The small number of cases (14 patients) and the use of the single criterion of intraluminal invasion for tracheal and esophageal involvement led to the detection of tracheal involvement in 50% (1 of 2) and esophageal involvement in 0% (0 of 1) of cases by MR imaging.
In a study of 22 patients with periesophageal neck masses, Roychowdhury et al16 indicated that a circumferential mass of >270° or focal T2 signal intensity abnormality of the esophageal wall on MR imaging suggests the presence of esophageal invasion with a specificity of 100% and 86%, respectively. On the other hand, an intact fat plane, absence of wall thickening, and no T2 wall-signal-intensity abnormality imply that the esophagus is not invaded, with a sensitivity of 100% for all 3 criteria.
In a case control study by Chen et al,17 CT and MR imaging of 78 patients with head and neck cancer was retrospectively analyzed. According to pathology and follow-up findings, patients were divided into a case group (N = 32) with esophageal inlet invasion and a control group (N = 46) without invasion. The distance between the posterior aspect of the cricoid cartilage and the anterior aspect of the vertebra (d-CV) was measured and compared between the 2 groups. The authors indicated that a d-CV of greater than 1.0 cm is an optimal criterion for esophageal inlet invasion by advanced head and neck carcinomas, with a statistical significance between the 2 groups. Accuracy was 79% and 80% on CT and MR imaging, respectively.
van den Hoed et al18 found a positive correlation between CT prediction of unresectability based on mediastinal involvement and patient outcome in 85 patients with esophageal carcinoma. Criteria used for the diagnosis of aortic invasion were 1) obliteration of peri-aortic fat and 2) an angle of contact >45° with the aorta. Median survival was 21 and 8 months, respectively, for tumors considered CT resectable or unresectable.
Koda et al19 compared the accuracy of intra-aortic endovascular sonography (IES) with that of CT in the diagnosis of aortic invasion in 28 patients with esophageal carcinoma who subsequently underwent surgical exploration. A criterion of obliteration of the outer hyperechoic layer of the aorta was used to diagnose invasion on sonography, whereas a contact angle of at least 90° with loss of periaortic fat was considered positive for invasion on CT. Accuracy was 100% for the presence of invasion by using IES and 89% for CT.
Endoscopic ultrasonography (EUS) is an imaging technique that is gaining popularity in esophageal cancer staging. Its great value relies on the ability to visualize in detail the 4 layers of the esophagus as well as the outer periesophageal fat. In a study by Wildi et al,20 17 patients presented with lower neck masses and suspected esophageal invasion on CT and were evaluated by EUS, which demonstrated esophageal invasion in 4 patients and pleural invasion in 1 patient. The remaining 12 patients (71%) showed no visible invasion on EUS, with a resultant change in the plan of management.
Criteria with Respect to Surgical Planning
Laryngeal Cartilage Invasion
A number of European groups have studied the issue regarding the presence or absence of laryngeal cartilage infiltration.21–30 The latest AJCC criteria for laryngeal cartilage invasion have stipulated a difference between the presence of cortical invasion of the inner margin of the thyroid cartilage (“minor thyroid cartilage invasion… . eg, inner cortex” as T3 cancer) versus through and through infiltration of laryngeal cartilage (“invades through the thyroid cartilage” as T4a cancer) (Table 1). Recent radiation therapy studies have suggested that limited cartilaginous invasion can be cured through localized radiation therapy with or without chemotherapy. Therefore, the previous adage that cartilage invasion precluded radiation therapy because of the high rate of recurrence or chondronecrosis has been altered during the past 5 years. Nonetheless, through and through cartilage invasion remains 1 of the primary indicators for surgical resection of the laryngeal carcinomas (Fig 4). To that end, these European groups have investigated extensively the criteria for the presence or absence of cartilage invasion by using CT and MR imaging criteria.
Gross infiltration of the laryngeal cartilage. Erosion of the right side of the cricoid cartilage (long arrow) is well demonstrated in this patient with laryngeal carcinoma with subglottic extension. Additionally, the anterior margin of the right thyroid cartilage (short arrow) appears to be invaded.
Agada et al31 examined data from 38 patients with SQCC of the larynx. When comparing CT staging with histopathology, they found that 45% of the patients were erroneously overstaged to T4 on the basis of laryngeal cartilage invasion as judged by the radiologic sign of cartilage sclerosis.
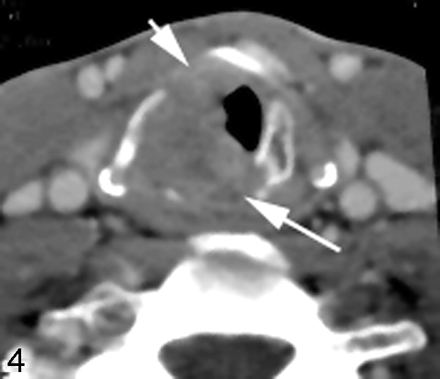
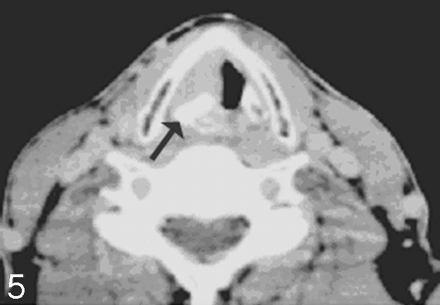
Becker et al26 looked at several CT laryngeal imaging findings that could suggest cartilage infiltration including 1) extralaryngeal tumor, 2) sclerosis, 3) tumor adjacent to nonossified cartilage, 3) serpiginous contour, 4) erosion or lysis, 5) obliteration of marrow space, 6) cartilaginous blowout, and 7) bowing. Although the authors were able to identify several criteria that had either a high sensitivity or a high specificity, no 1 criterion had both sensitivity and specificity over 70% with regard to the thyroid cartilage. The findings were a little more optimistic with regard to the presence of invasion of cricoid or arytenoid cartilage, in which sclerosis and through and through involvement were the most accurate criteria (Figs 4 and 6).
Laryngeal cartilage sclerosis. Axial postcontrast CT scan at the level of the true vocal cord shows sclerosis of the right arytenoid cartilage (arrow), associated with thickening of the true vocal cord. This finding is suggestive of cartilaginous invasion or perichondrial reaction.
Arytenoid sclerosis in laryngeal carcinoma. Axial CT scan with bone windows shows a sclerotic right arytenoid cartilage (arrow) in a patient with true vocal cord cancer. Despite the sclerosis, there was no evidence of cartilaginous invasion on histology.
The reason that the thyroid cartilage is the most difficult one of the larynx to assess is because it often has areas of chondrification and ossification in contiguity with each other. Although ossified cartilage has a different attenuation than tumor and therefore is more readily assessed, chondrified cartilage may be isoattenuated to the adjacent neoplasm, making the assessment that much more difficult. Although the resolution of the multidetector CT (MDCT) is far superior to that of MR imaging currently, the soft-tissue contrast to discriminate between the 2 types of tissue is the weakness of MDCT.
The MR assessment of cartilaginous invasion requires evaluation of thin-section T2-weighted fat-suppressed scans and thin-section T1-weighted post–gadolinium-enhanced scans. MR imaging studies have suggested that high signal intensity within the boundaries of the cartilage on the T2-weighted fat-suppressed scans and contrast enhancement into the cartilage, demonstrated as high signal intensity against a dark background of suppressed ossified fat, low-signal-intensity bone of the ossified cartilage, and low-signal-intensity chondrified cartilage, are indicative of infiltration. Using these criteria, Zbaren et al, Castelijns et al, and Becker et al21–25 demonstrated a high sensitivity though low specificity for cartilage infiltration with MR imaging.
CT criteria have high specificity but low sensitivity and a mean accuracy of approximately 75%–80% versus MR imaging, in which there is a high sensitivity but low specificity and accuracy rates of, on average, 80%–85%. The implications are quite important; a false-positive prediction of cancer infiltrating cartilage on MR imaging may result in inadvertent removal of the larynx when the cancer might have been curable with larynx-sparing medical therapy or limited surgery. The cosmetic and functional deformities of total laryngectomy have long-ranging implications as described previously. On the other hand, using a study that has low sensitivity (CT) could lead to deferral of surgery, incomplete surgery, or inappropriate radiation therapy with either tumor remaining or the inability to cure the patient.
Nishiyama et al32 studied the value of single-photon emission tomography (SPECT) compared with that of radiologic examination (CT and/or MR imaging) in the detection of cartilage invasion in patients with laryngohypopharyngeal cancer. They suggested that superimposed early bone and tumor dual-isotope SPECT images may be sufficient in the diagnostic evaluation of cartilage invasion by superimposing tumor location (from tumor SPECT) onto the osseous structures (shown on bone SPECT) with an accuracy of almost 90% (17 of 19 cases).
Pre-epiglottic Fat Invasion
One of the factors determining the potential for the performance of conservation laryngeal surgery for cancer is the extent of invasion of the pre-epiglottic fat.33,34 When there is extensive pre-epiglottic fat infiltration, the hyoid bone is often at risk for infiltration. The hyoid bone is critical for the supracricoid surgical laryngeal conservation procedure, which requires a pexy between the hyoid bone and the cricoid cartilage to allow reconstruction. This pexy procedure is required to pull the incised portions of the larynx superiorly so that the arytenoid cartilage can appose the tongue base with or without the epiglottis to create laryngeal airway closure during swallowing. When the epiglottis is preserved, the procedure is called a cricohyoidoepiglottopexy, whereas reconstruction without the epiglottis is called cricohyoidopexy. This is a procedure that can be performed for tumors with limited thyroid cartilage invasion be it from the supraglottic or glottic primary sites.
The presence of pre-epiglottic fat infiltration was evaluated with MR imaging by Loevner et al.35 The assessment was best made on sagittal T1-weighted or axial T1-weighted scans, in which the high-signal-intensity fat is an excellent background with which to evaluate neoplastic infiltration. Using the substitution of soft-tissue signal intensity for the pre-epiglottic fat, the authors studied 40 patients with supraglottic laryngeal or pharyngeal carcinomas in which pre-epiglottic fat invasion was suspected. The accuracy rate of the MR evaluation was 90% with 4 false-positive cases, which were related to either partial volume averaging, peritumoral edema extending into the pre-epiglottic fat, paraglottic fat infiltration that was mistakenly called pre-epiglottic fat, and minor salivary gland ectopic tissue mistaken for neoplasm.
The extent of pre-epiglottic fat infiltration is not just important from the standpoint of surgical options.33 In addition, it has been shown that pre-epiglottic fat infiltration by neoplasm increases the risk of cervical adenopathy of the primary tumor. When the pre-epiglottic fat is infiltrated from a supraglottic laryngeal carcinoma, there is also the possibility that a cuff of tissue from the tongue base may also need to be resected. This resection has the attendant morbidity of swallowing dysfunction and poor airway preservation.
Dural Infiltration
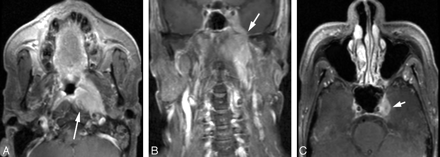
Another issue with regard to sinonasal cavity cancer or other cancers that may infiltrate the skull base is the presence of dural infiltration (Fig 7). Dural infiltration can be considered a preliminary step before potential parenchymal invasion. Therefore, its detection is critical, particularly when determining if craniofacial surgery is required. Craniofacial surgery requires the combined efforts of a neurosurgeon/skull base surgeon as well as a head and neck surgical oncologist.
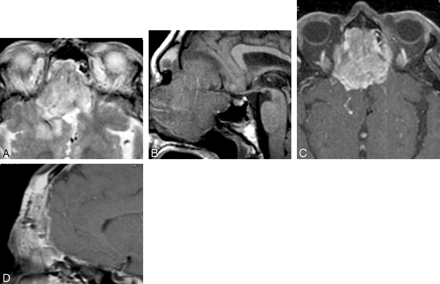
Dural and intracranial extension of mass. A, Axial T2-weighted scan demonstrates soft-tissue mass infiltrating the anterior cranial fossa with associated edema of the right frontal lobe. B, Parasagittal T1-weighted scan shows the gross infiltration across the cribriform plate to involve the anterior cranial fossa with massive involvement in an extra-axial location. C, Postcontrast fat-suppressed T1-weighted scan shows intracranial extension as well as dural infiltration and pial extension of tumor adjacent to the midline. D, A different patient with adenocarcinoma of the paranasal sinuses shows considerable thickening of the dural greater than 5 mm, indicative of dural infiltration, confirmed at surgery.
Dural infiltration versus reactive change, even for tumors such as meningiomas, can be problematic to differentiate on the basis of imaging findings. Although some studies have suggested that the dural tail of a meningioma is largely due to neoplasm, others have suggested that the dural tail may, in part, be due to reactive vascular tissue in the dura. This can be assessed at frozen section; however, most surgeons have adopted the philosophy of removing the entire dural tail of a meningioma rather than potentially leaving tumor cells behind.
With respect to dural infiltration by a malignant neoplasm, it is critically important to be able to give a patient a reasonable sense of the likelihood of a complete resection once a combined craniofacial approach is considered. To go through the surgery unnecessarily or to opt to not go through the surgery and potentially leave residual surgically treatable tumor behind is a difficult decision to make.
Eisen et al36 evaluated 23 patients in whom dural infiltration by facial head and neck neoplasm was suspected. The authors measured the thickness of the dura, classified the dura in terms showing of nodular versus linear enhancement, and also assessed the presence of pial enhancement. Although there were a limited number of positive cases in this study, the authors found that when the linear dural enhancement was greater than 5 mm in thickness, the likelihood of neoplastic infiltration of the dura was very high. If there was nodularity to the enhancing dura or if there was pial enhancement, cancerous infiltration was present histopathologically. However, linear dural enhancement less than or equal to 5 mm in thickness correlated with a benign histology in the study. The finding of this study that thin linear enhancement need not suggest neoplastic infiltration has been reproduced in numerous settings. Please note that there are numerous reasons for linear dural enhancement of the meninges beyond neoplasm, including infectious, vascular, and inflammatory etiologies.
Bone Infiltration
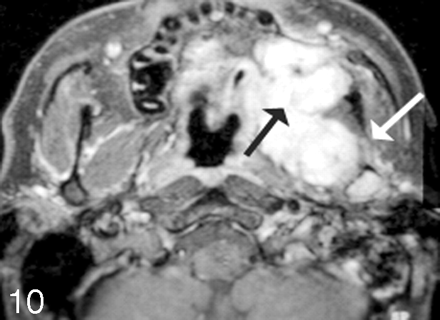
The presence of infiltration of the skull base and/or the facial bones is also critical to the assessment of the extent of surgery required for resection of tumors. One site that has extensive importance with respect to the surgical approach to tumor resection is infiltration of the mandible or maxilla from oral cavity or oropharyngeal carcinoma (Figs 8–10). The reconstruction of the mandible, if it has to be completely or partially resected, often requires bone grafting from another site. This is accomplished with simultaneous surgical teams, one harvesting and preparing the donor site while the other is involved in the surgical resection of the cancer. An unexpected discovery of bony disease while in the operating room renders the reconstruction procedure problematic. Planning for the possibility of a complicated reconstruction is critical to achieve the best functional outcome for the patient.
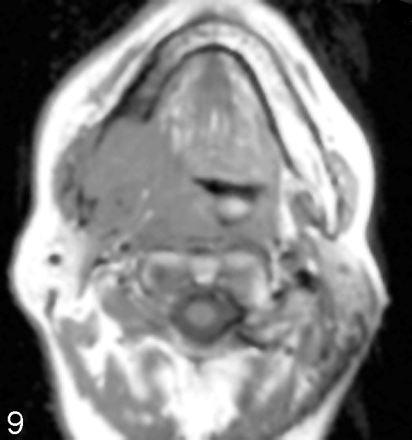
Post–gadolinium-enhanced scan through the floor of the mouth demonstrates a contrast-enhancing mass (arrow) infiltrating the right side of the mandible and extending into the subcutaneous tissue. There is an associated mass at the right lateral base of the tongue. Note infiltration of the mandible extends across the midline.
Infiltration of the mandible with retropharyngeal spread of tumor. Axial T1-weighted scan demonstrates a large mass emanating from the right palatine tonsil extending along the floor of the mouth posteriorly to infiltrate the right side of the mandible. Note that the signal intensity of the right side of the bone marrow is dark, replacing the bone marrow fat. There is effacement of the retropharyngeal fat on the right side, with tumor abutting the longus colli/capitis muscle complex.
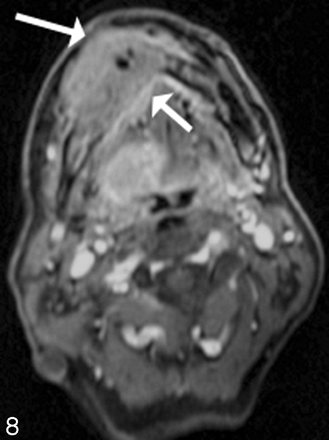
Sarcoma growing into the left maxilla. Postcontrast fat-suppressed images demonstrate soft-tissue enhancement emanating from the retromolar trigone region (black arrow), with growth into the posterior margin of the left maxilla and abutting the coronoid region of the left mandible. There is likely perineural spread as seen on the axial scan with enhancement into the inferior alveolar canal (white arrow).
Mandibular Invasion
Brown37–41 described the mechanisms of cancer invasion of the mandible in a detailed report based on an extensive review of 57 related articles. He indicated that the route of tumor entry to the mandible is at the point of abutment, which is often the junction of the attached and reflected mucosa below the crest of the alveolar ridge, with a positive relationship between the infiltrative pattern of the disease and the size of the primary tumor. He noted that preferential tumor spread along the inferior alveolar nerve or the bone marrow is unusual, and this characteristic argues against the inclusion of the neurovascular bundle in marginal resections of the mandible.
Chung et al42 investigated the ability of MR imaging to predict neoplastic infiltration of the mandible in patients with oral cavity and oropharyngeal carcinomas. The authors found that MR imaging had high sensitivity but relative low specificity in this arena. The authors used findings of replacement of the normal high-intensity bone marrow at T1-weighted scanning, the presence of cortical erosions and contrast enhancement in the cortex or marrow, or high signal intensity on T2-weighted scanning in the bone marrow to identify neoplastic infiltration. False-positive findings were identified in those individuals who had undergone recent dental extractions or radiation therapy or who had infectious inflammatory odontogenic disease. In these cases, the marrow signal intensity often was abnormal, and there was abnormal contrast enhancement present. In those individuals who had undergone recent extractions, the presence of cortical erosion from neoplasm could not be distinguished from tooth-extraction defects. Osteoradionecrosis also accounted for a false-positive study. However the MR imaging demonstrated that no false-negative findings existed (100% sensitivity). This included a total of 22 patients who had mandibular resections.
Using the same criteria of MR imaging in a larger group of patients43 with oral or oropharyngeal SQCC, Bolzoni et al44 identified mandibular invasion in 16 patients, with the tumor confined only to the cortex in 6. They reported a sensitivity, specificity, and accuracy of 93%, 93%, and 93% respectively.
With the advent of 0.5-mm MDCT scanning of the head and neck, subtle erosions of the cortex of the mandible can readily be ascertained by axial scanning with multiplanar reconstructions. New literature has suggested that the sensitivity and specificity of CT for mandibular invasion by neoplasm has improved with the higher resolution afforded by MDCT. Nonetheless, it is expected that the sources for false-positive studies (ie, dental extraction defects, radiation fibrosis, and osteoradionecrosis) in the MR imaging arena might still provoke an inaccurate CT scan. Positron-emission tomography (PET) scanning may be of some utility in these settings; however, it has not been extensively investigated to date.
Brockenbrough et al45 evaluated the diagnostic accuracy of DentaScan (dental CT software) in predicting mandibular invasion in 36 patients with SQCC of the oral cavity by using histopathology as a gold standard. With DentaScan, a thin axial dataset of images can be reformatted into multiple illustrative panoramic and cross-sectional views that allow better ultrastructural visualization of the mandible without intravenous contrast administration. Using a section thickness of 1–1.25 mm, the authors could identify bone invasion in 21 of 22 patients (sensitivity, 95%). However, they had 3 false-positive results (specificity, 79%). The relatively low specificity was partly related to the small number of patients without bone invasion (14 of 36).
Compared with CT, MR imaging appears less specific in assessing the presence and extent of mandibular invasion. In a recent work by Imaizumi et al,43 51 patients with oral SQCC were evaluated by using MR imaging and axial CT for the presence and extent of mandibular invasion. In 35 patients in whom axial (5-mm-thick) CT was difficult to evaluate, a dedicated dental CT (DentaScan) examination was performed by using 1-mm-thick images. The specificity of CT was higher than that of MR imaging in all aspects of evaluation, including mandibular cortical invasion (88%, 54%), bone marrow involvement (88%, 81%), and inferior alveolar canal invasion (96%, 70%) for both CT and MR imaging, respectively.43 The 12 false-positive diagnoses of mandibular cortical invasion by MR imaging were due to a chemical shift artifact that obscured the cortical line. MR imaging also had 14 false-positive diagnoses of inferior alveolar canal invasion, which was explained by the presence of inflammation surrounding the tumor, which shows similar signal intensity.
Jungehulsing et al46 compared different imaging techniques, including SPECT, with histopathology for the prediction of mandibular invasion in 35 patients with oral SQCC. With a semiquantitative assessment of technetium Ic99m methylene diphosphonates uptake by the affected part of the mandible compared with an unaffected part, they found that SPECT could correctly predict 11 of 12 cases of mandibular invasion with no false-positive results.
PET/CT fusion is a relatively new technology that combines the precise structural information of CT with the sensitive metabolic information of PET into 1 diagnostic image. Babin et al47 evaluated mandibular invasion in 8 patients with oral/oropharyngeal carcinoma by using PET/CT fusion. They proved the technique to be 100% sensitive and 83% specific compared with histopathology. Findings were interesting, but further larger studies may be needed to confirm those preliminary results.
Skull Base Invasion
In a study from Korea, Roh et al48 retrospectively analyzed the data of 119 patients with nasopharyngeal carcinoma (NPC). Skull base invasion was reported in 46 (38.6%) patients. In terms of prognostic values, the authors recommended further subdivision of NPC with skull base invasion into 4 groups in ascending order of prognostic importance: 1) simple skull base erosion, 2) minimal involvement of either anterior or posterior cranial nerves, 3) multiple involvement of both groups of cranial nerves, and 4) intracranial extension.
Yu et al49 demonstrated different CT features in 58 patients with maxillofacial tumors invading the skull base. The main manifestations observed on CT were foraminal enlargement, bone thinning, erosion, and displacement. The following structures were involved in descending order of frequency: the roof of the pterygoid process of the sphenoid bone, the greater wing of sphenoid, the sphenoid body and sinus, the petrous apex, the clivus, and the articular surface of the temporal squamosa.
Xie et al50,51 compared MR imaging with CT in 63 patients with NPC. They found that MR imaging was able to detect skull base erosion more easily than CT (23 and 15 cases for MR imaging and CT, respectively) with a noticeable impact of MR imaging on the accuracy of the staging of NPC.
The same parameters for infiltration of the mandible can be applied to other locations at the skull base. Once again, replacement of bone marrow high signal intensity on T1-weighted scans, erosion of the bone as demonstrated by contrast-enhancing tissue against a fat-suppressed background, and high-signal-intensity marrow edema have been used in this regard. Unfortunately, there are numerous pathologic sources of low signal intensity in the bone marrow including chronic anemias, HIV infection, hematologic disorders, fibrosis dysplasia, Paget disease, osteomalacia, vitamin deficiency sources, as well as postherapeutic effects.52 Recent traumatic injuries to the bone may also demonstrate enhancement, high signal intensity on T2-weighted scans, and replacement of high-signal-intensity bone marrow fat on T1-weighted scans. This has been equally problematic when evaluating the spinal column.
Stambuk et al53 demonstrated a model of skull base invasion with NPC. They evaluated 11 children (12–17 years of age) who presented with NPC. Because pediatric NPC is generally not suspected clinically until late in the disease process, they found that all patients except 1 (91%) had skull base invasion by the time of radiographic evaluation (CT and/or MR imaging). Widening of the petroclival fissure was present in 8 (73%) patients. The tumor had extended to involve the pterygopalatine fossa in 2 (18%), and the sphenoid sinus in 3 (27%) cases. Clival invasion was seen as abnormal high T2 signal intensity on fat-saturated MR imaging.
Some investigators have advocated the use of diffusion-weighted scanning and calculating apparent diffusion coefficient (ADC) values to distinguish neoplastic infiltration (with its lower ADC) versus non-neoplastic infiltration (with higher ADC values).54,55 This has been applied with both diffusion-weighted imaging, diffusion line scanning, and calculations of ADC values for spinal compression fractures and has resulted in modest levels of accuracy. These same perimeters may be applied to the skull base.
Perineural Spread or Tumor
Another phenomenon that may affect the therapeutic decision making in dealing with head and a neck cancer is the presence of perineural spread of tumor. In some cases perineural spread of tumor refers to tumoral infiltration of the nerve itself (Fig 10) as opposed to spread on the nerve by using the nerve as a scaffold for neoplastic spread. Perineural spread in the context of this article will incorporate both explanations as well as tumoral spread through the neural foramina of the skull base.
Many theories have been proposed to understand the exact mechanism of perineural spread of tumors. Tumor cells are known to spread along the perineural connective tissue or the endoneural plane. Neural cell adhesion molecule (N-CAM) expression has been shown to be positively correlated with perineural spread in a variety of tumors. Gandour-Edwards et al56 demonstrated the expression of N-CAM in 93% (14 of 15 patients) of adenoid cystic carcinomas that showed perineural spread. What is most interesting, Vural et al57 found the same percentage (93%, 38 of 41 patients) of expression of N-CAM 3 years later in SQCC with perineural spread.
Perineural spread on CT is demonstrated by the enlargement of the neural foramina and/or soft-tissue infiltration of the fat adjacent to or within the neural foramina.58–66 Soft-tissue infiltration of the foramen rotundum, for example, can be assumed to be neoplastic infiltration in most CT settings, given a tumor with a propensity to access the second division of cranial nerve V. In a similar vein, foramen ovale infiltration (Fig 11) implies a third division of cranial nerve V infiltration. Contrast-enhanced CT rarely shows actual enhancement of tumor along the nerve; however, this can be readily identified on fat-suppressed T1-weighted postcontrast MR imaging. The CT evidence is by inference, whereas there can be direct visualization of enhancement of a nerve and tumor around the nerve with MR imaging. MR imaging may also demonstrate infiltration of the fat of the foramina at the skull base or the trigeminal ganglion on the basis of T1-weighted scanning demonstrating the soft-tissue low-intensity signal that replaces the white-signal-intensity fat.
Nasopharyngeal carcinoma with infiltration of the prevertebral musculature, skull base, and cavernous sinus. A, Postcontrast fat-suppressed T1-weighted scan shows a large nasopharyngeal carcinoma, which demonstrates contrast enhancement of the prevertebral musculature on the left side (arrow). B, Note the growth through the foramen ovale (arrow) into the Meckel cave and cavernous sinus on the left side via cranial nerve V. C, The cavernous sinus extension on this axial postgadolinium fat-suppressed scan is well demonstrated (arrow). There may also be enhancement in the pterygopalatine fossa on the left side.
Although adenoid cystic carcinoma is the histologic neoplasm most commonly associated with perineural spread, at a rate of approximately 60%, SQCC, basal cell carcinoma, mucoepidermoid carcinoma, lymphoma, neurotropic melanoma, rhabdomyosarcoma, and metastasis may certainly demonstrate this phenomenon.
Adenoid cystic carcinoma populates salivary gland neoplasms; therefore, infiltration of cranial nerves V and VII is the most commonly seen. Spread along cranial nerve XII, cranial nerve VII, and cranial nerve V by oral cavity or retromolar trigone cancers is another commonly identified process with SQCC. In fact, the T4b classification for oral cavity and oropharyngeal carcinoma specifically addresses invasion of the lateral pterygoid muscle, the pterygoid plates (bone and nerves of the pterygopalatine fossa), the skull base, and the carotid artery.
Chang et al67 identified 8 cases of malignant melanoma at 2 institutions that had MR imaging evidence of perineural spread with confirmatory tissue sampling. Five cases (63%) were desmoplastic melanomas. Enhancement of at least 1 branch of the trigeminal nerve was demonstrated in all cases and at least 1 other cranial nerve in 5 cases. Other findings included abnormal enhancement and soft-tissue thickening of the cavernous sinus, Meckel cave, and/or the cisternal portion of the trigeminal nerve.
There are few case reports in the literature describing lymphomatous infiltration of the mandibular canal (along the inferior alveolar nerve). Yamada et al68 reported a case of non-Hodgkin lymphoma, which had slowly grown into the mandibular canal with continuous dilation to an average of 15-mm width with peripheral bone sclerosis and no destruction.
Moorjani and Stockton69 reported a very rare case of desmoplastic fibroma of the mandible with perineural spread along the third division of trigeminal nerve, which appeared enlarged and showed abnormal enhancement along its course in the masticator space, through the wide foramen ovale, and in Meckel cave.
The cranial nerves to the orbits (cranial nerves III, IV, and VI to the extraocular muscles and V for sensation) are uncommonly infiltrated with neoplasm; however, infiltration may be seen as enhancing nerves in patients with plexiform schwannomas, lymphoma, and sarcoidosis. Skin cancers over the anterior face may infiltrate all 3 divisions of cranial nerve V and can represent a significant therapeutic predicament for someone with what was initially expected to be a relatively small skin lesion.
Imaging studies of perineural spread include works by the MD Anderson Cancer Center group (Ginsberg, Ginsberg and Eicher, Ginsburg and DeMonte, and Ginsburg et al58,59,62–65), which have shown detection rates of approximately 70%. Certainly microscopic disease that occurs along the cranial nerves may escape imaging surveillance. The propensity for “skip regions” in what appears to be noncontiguous tumor cells presents another daunting task for the radiologic evaluation. Rarely, intracranial spread with enhancement of the cisternal portion of the cranial nerves may be visualized. When one is suspecting microscopic perineural spread, it is difficult for the radiation therapist to assign portals for the extent of the tumor. By incorporating a greater amount of the intracranial contents and brain stem, the radiation oncologist risks the toxicity to those cranial nerves and/or to the visual apparatus with respect to the optic nerves and optic chiasm. Again, quite often the issue is “At what cost, cure?” This problem is especially apparent with adenoid cystic carcinoma, which is a relatively indolent malignancy in which, even without therapy, long-term survival measured in terms of decades may occur. The added benefit of therapy, though not inconsequential, is also not overwhelming with respect to improved 20-year survivals.
Gandhi et al70 described, in a review article, the different patterns of perineural spread on MR imaging. Direct signs included cranial nerve enlargement, irregularity, excessive enhancement, and foraminal enlargement with destruction in advanced disease. The authors also addressed the importance of careful observation of the normal intraforaminal fat pads on T1-weighted scans, which can be obliterated with tumor infiltration. Muscle denervation is an indirect sign of cranial nerve involvement. Swelling, abnormal T2 signal intensity, and enhancement can be seen with acute-subacute denervation, whereas chronic muscle denervation results in fatty replacement and muscle atrophy.
Orbital Invasion
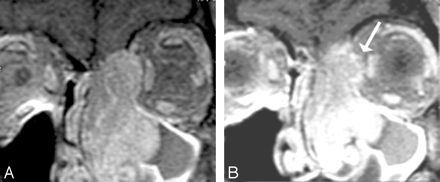
Sinonasal carcinomas are dangerous from the standpoint of their proximity to the orbit as well as the skull base (Fig 12). Orbital infiltration is generally limited by the tough periorbita, which functions as an excellent barrier to the spread of neoplasm, similar to that of the periosteum. Unfortunately, the periorbita is not readily identifiable even in high-resolution MR imaging or thin-section CT. Peritumoral edema can lead to abnormal signal intensity within muscles and abnormal contrast enhancement of muscles, which simulates neoplasm on MR imaging. This potential for false-positive results is less of an issue with CT.
A and B, Orbital infiltration. Postcontrast fat-suppressed T1-weighted scans show soft-tissue infiltration in the superior medial aspect of the left orbit, with irregular margins in the extraconal fat. Although there are portions of the tumor in which there still appears to be periosteum containing the tumor, the irregular margins seen more anteriorly (arrow) suggest infiltration requiring orbital surgery.
Invasion of the orbits by head and neck tumors can occur either by using the route of skull base foramina and sinuses or by direct extension through the orbital bone (Fig 13). In a large study of 562 patients with nasopharyngeal carcinoma, Luo et al71 identified orbital involvement by using CT and/or MR imaging in 18 patients. The route from the pterygopalatine fossa and the inferior orbital fissure into the orbital cavity was the most common route of invasion (n = 13), followed by ethmoid and/or sphenoid sinuses into the orbit (n = 4). In 1 patient, the exact route could not be identified.
Orbital infiltration. A, Soft tissue is seen in the posterior left orbit in this patient with metastatic disease to the bone. The infiltration of the intraconal fat suggests the need for orbital exenteration surgery, if surgical treatment is even contemplated. B, Note the erosion at the junction of the posterior left orbit with the frontal bone. C, One can see that there is dural extension of the tumor accompanying the orbital infiltration (arrows).
Eisen et al72 examined the accuracy of CT and MR imaging in predicting tumor invasion of the orbit. Nineteen preoperative CT scans and 17 preoperative MR images from patients with cancer at risk of orbital invasion were retrospectively reviewed in correlation with pathologic and intraoperative assessment. Eleven criteria involving the tumor, orbit, and nasolacrimal fossa were used in each study. Tumor adjacent to the periorbita was the most sensitive predictor of orbital invasion (90%) for both CT and MR imaging. The highest positive predictive values were achieved with extraocular muscle involvement manifested as high signal intensity on T2-weighted scans or enhancement on MR imaging (100%) and orbital fat infiltration (80% on MR imaging, 86% on CT). No one criterion was more than 79% accurate in predicting orbital invasion, whereas nasolacrimal invasion was predicted with an accuracy of 89%. CT was more accurate than MR imaging in 7 of 9 criteria.
Brachial Plexus Infiltration
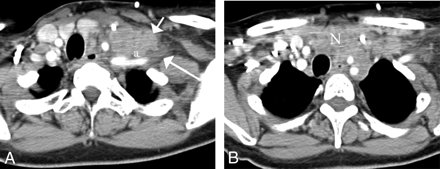
Related to perineural spread of tumor is direct invasion of the brachial plexus. Because head and neck surgical oncologists are less comfortable with the implications of resecting tumor from the nerves that feed the upper extremities, these discussions are often made in consultation or in collaboration with neurosurgeons. In most instances, the head and neck surgeon will deem the patient inoperative if the patient has any symptoms whatsoever referable to the brachial plexus or if the anterior scalene muscle is infiltrated by the neoplasm. Anterior scalene muscle infiltration is based on loss of the normal attenuation appearance and fatty striations of the muscle on CT, loss of the fat plane between the middle-posterior scalene complex and the anterior scalene muscle through which the brachial plexus passes, or direct displacement of the visualized portion of the brachial plexus (Fig 14). This is better identified with MR imaging, in which high signal intensity on T2-weighted imaging in the muscle, gadolinium enhancement of muscle, or enhancement or abnormal T2W signal intensity along the brachial plexus roots, trunks, divisions, cords, and branches is seen.
A, Nodal metastases from breast cancer (short white arrow) are seen to infiltrate the anterior scalene muscle (a) and the hypodense brachial plexus (long white arrow) on this enhanced axial CT image. A tongue of nodal disease indents the plexus beyond the anterior scalene muscle.
B, This same nodal mass (N) infiltrates the anterior mediastinum and abuts on the supraortic vessels in the left.
In a retrospective review of the clinicopathologic data of 71 patients with cancer and brachial plexopathy, Thyagarajan et al73 indicated that the presence of a mass adjacent to the brachial plexus was highly predictive of tumor infiltration and was the most useful feature in distinguishing infiltration from radiation plexopathy; increased T2 signal intensity was commonly seen in both groups.
Ohta74 reported a rare case of brachial plexus infiltration by non-Hodgkin lymphoma. Findings included soft-tissue masses with abnormal T2 hyperintensity signal intensity along the brachial plexus. Scintigraphy was superior to MR imaging in detecting the abnormality, showing increased uptake of gallium-67 along the course of the brachial plexus.
Recently, Graif et al75 used ultrasonography to evaluate 28 patients with different varieties of brachial plexus pathology. Most of the patients underwent surgical confirmation. On scanning along the nerve course from its exit at the vertebral foramina down to the axilla and in the presence of some technical limitations, the authors could depict different patterns of sonographic abnormalities. Among 10 patients with secondary tumors, 6 patients with primary tumors, and 6 patients with associated radiation fibrosis, findings were categorized as either focal hypoechoic masses adjacent to the nerves with fusiform nerve thickening or diffuse nerve thickening. Despite a false-negative rate of 29% of cases, some of these cases showed tumors that were small (<12 mm) posttraumatic lesions. Ultrasonography may be useful as part of the preoperative evaluation of brachial plexus infiltration.
Resectability Issues in Patients Who Have Undergone Previous Treatment
Combined chemoradiation regimens for advanced-stage head and neck cancer have gained popularity during the last decade. The advent of new treatment protocols and novel chemotherapeutic agents, along with refinements in the field of radiation oncology, have made it possible to treat advanced-stage head and neck cancer for cure while avoiding the morbidity of surgical intervention. Tumors deemed unresectable are now being treated in this fashion as well, with curative intent. In the last few years, postoperative adjuvant chemoradiation has been recommended for patients who have undergone primary surgical intervention with histopathologic criteria that are associated with a high risk of locoregional recurrence.76
The evaluation of a patient who has been previously treated for advanced head and neck cancer and now has documented cancer recurrence or persistence poses many diagnostic and therapeutic challenges. These patients typically can no longer receive any further radiation and chemotherapy at curative doses. Surgical salvage rates are poor. Early detection of tumor recurrence or persistence is a key factor in improving disease control rates and survival rates for patients undergoing surgical salvage treatment. The same issues that have been elucidated earlier with regard to resectability still apply. The problem with this patient subset is in distinguishing treatment effect from tumor recurrence or persistence.
PET/CT fusion technology has been especially helpful in this patient subset. Surgery and chemoradiation therapy both result in anatomic changes that are difficult to characterize purely by conventional imaging (CT, MR) alone. Conventional imaging is typically obtained within a 2- to 3-month period after completion of treatment to establish a baseline for which all future studies will be compared. If this baseline scan or any future scan demonstrates any change or irregularity that raises the suspicion for cancer recurrence or persistence, then PET/CT may be ordered to help characterize those changes on the basis of the degree of metabolic activity.77 If tumor recurrence or persistence is still suspected, then a detailed evaluation with the patient under anesthesia with possible biopsy is warranted.
Defining the extent of tumor recurrence or persistence in this patient population is key to the surgeon and patient in determining resectability and the likelihood of surgical salvage with any meaningful long-term survival.
Summary
Imaging can be very helpful in the determination of resectability for head and neck cancers. The decision to attempt extensive and significantly morbid resections for tumor control must rest with the surgeons and patients and should be, in part, based on the most accurate imaging techniques available. MR imaging benefits from a high sensitivity but is prone to false-positive findings, a failure that may be addressed through the judicious use of PET/CT. High-resolution MDCT with multiplanar reconstructions is another alternative in addressing the critical issues of vascular encasement, skull base or perineural infiltration, cartilaginous invasion, orbital involvement, mediastinal (including trachea and esophagus) spread, and dural disease. Imaging is less successful in issues of prevertebral fixation and early cartilaginous invasion.
Acknowledgments
Laurie Loevner is credited for numerous contributions to the concepts in this manuscript as well as the head and neck literature on cancer resectability.
Footnotes
Dr. Khaled Gad’s work on this topic was supported in part by the Egyptian government.
References
- Copyright © American Society of Neuroradiology