Abstract
BACKGROUND AND PURPOSE: Current organ-preservation regimens for upper aerodigestive tract squamous cell carcinoma (SCCA) require endoscopic procedures under general anesthesia to evaluate the tumor response. The purpose of our study was to determine whether CT perfusion (CTP) parameters correlate with response to induction chemotherapy as assessed by endoscopy under general anesthesia.
METHODS: Nine patients with advanced (stage 3 or 4) SCCA of the oropharynx were enrolled in a nested phase 2 prospective trial in which induction chemotherapy was used to assess the tumor response. Patients underwent direct laryngoscopy and CTP before and 3 weeks after one cycle of induction chemotherapy. The outcome variables were the surgeon’s estimate of tumor volume during endoscopy with biopsy under anesthesia and CTP parameters (capillary permeability (CP), blood volume (BV), blood flow (BF), and mean transit time (MTT)). Wilcoxon rank sum analysis was used to correlate the baseline values of BF and BV with response to induction chemotherapy. Comparison of agreement between the reduction in tumor volume and change in CTP parameters was performed by using kappa estimates.
RESULTS: Seven of 9 patients demonstrated ≥50% tumor volume reduction, representing positive response to induction chemotherapy. In the responder group, the following changes in mean pre- and postinduction chemotherapy values were noted: mean BF, 114.2 mL/100 g /min (preinduction) to 45.1 mL/100 g/min (postinduction); mean BV, 5.11 mL/100 g to 3.1 mL/100 g; mean CP, 25.6 mL/100 g /min (preinduction) to 18.3 mL/100 g / min (postinduction); mean MTT, 4.9 seconds (preinduction) to 8.0 seconds (postinduction). In the nonresponder group, the following changes were noted: mean BF, 56.9 mL/100 g/min to 75.9 mL/100 g/min; mean, BV 2.7 mL/100 g to 4.71 mL/100 g; mean CP, 24.1 mL/100 g/min to 23.7 mL/100 g/min; mean MTT, 4.3 seconds to 5.34 seconds. Higher baseline (pretherapy) values of BV showed significant correlation with endoscopic tumor response (P < .05). Reduction in the BV (by ≥20%) on follow-up studies also showed substantial agreement with clinical response as assessed with endoscopy (kappa = 0.73). The agreement between decreased BF, decreased CP, and increased MTT and clinical response was fair (kappa = 0.37).
CONCLUSION: These preliminary results show that deconvolution-based CTP technique offers potential for noninvasive monitoring of response to induction chemotherapy in patients with oropharyngeal cancers. Percentage reduction of BV is significantly correlated to endoscopic response to induction chemotherapy, though we acknowledge that the data correspond to short-term outcomes and long-term durability of response cannot be established. Nevertheless, validation of the use of deconvolution CTP parameters as predictors of tumor response may permit replacement of an invasive diagnostic procedure conducted under anesthesia currently used to assess response with noninvasive perfusion CT imaging.
The treatment of squamous cell carcinoma (SCCA) arising in the oropharynx has been evolving over the past decade. The former standard of care in the United States was surgery followed by radiation therapy. This approach led to survival rates between 25% and 50% in stage 3 and 4 disease. In addition, patients experienced significant difficulty with speech and swallowing after these procedures. In Europe, radiation was the standard of care. The survival rates were lower with single-technique therapy, but the patients tended to have better function with respect to speech and swallowing. Chemotherapy was introduced as both a radiosensitizer and as an induction regimen.1,2 Chemotherapy was shown to improve survival. Induction chemotherapy has been used in the treatment of laryngeal and oropharyngeal carcinomas.3,4 This approach continues to be used at present and involves administration of a single dose of chemotherapy as a technique to determine whether the tumor is responsive to chemotherapy. If the tumor is not responsive, the patient undergoes an alternative therapy such as surgery and radiation therapy. The underlying concept is based on the knowledge that SCCA is heterogeneous and, as a result, may respond differently to certain treatment regimens.
Part of the challenge in the logistics of this approach is determining the best way to assess the response to treatment. The gold standard is direct laryngoscopy with biopsy under general anesthesia. Endoscopic assessment of tumor volume under general anesthesia is performed, first as baseline for accurate tumor staging and subsequently for the assessment of response to therapy. Development of an alternative, efficacious, noninvasive method for assessment of tumor response could spare the patients the risk of repeated general anesthesia and invasive operative procedure. One such approach is anatomic imaging with CT or MR, but these can be difficult to interpret because of treatment-associated soft tissue edema.
Deconvolution-based CT perfusion (CTP) is a fast and robust imaging technique that is increasingly used as a clinical tool to help evaluate intracranial vascular disorders and as a research tool to help characterize intracranial mass lesions.5–7 This technique can assess physiologic parameters such as blood flow (BF), blood volume (BV), mean transit time (MTT), and capillary permeability surface area product (CP) and provides data that can be useful in the detection and characterization of entities such as tumor, infection, inflammation, and infarction.5 By using this technique, previous investigators have recently demonstrated increased BF, BV, CP, and reduced MTTs in head and neck SCCAs compared with adjacent normal structures.8
Tissue perfusion and local oxygen delivery are known to influence tumor response to nonsurgical therapies.9,10 By using a dynamic method for measuring CTP, Hermans et al demonstrated that CT-determined tumor perfusion is an independent predictor of local control in head-and-neck cancer treated by definitive radiation therapy, with or without adjuvant chemotherapy.11 The purpose of our study was to determine whether CTP parameters correlate with clinical response to induction chemotherapy as assessed by endoscopy under general anesthesia.
Methods
Patients
Nine patients with advanced (stage 3 or 4) SCCA of the oropharynx were enrolled in a prospective trial in which response to neoadjuvant chemotherapy was assessed. Inclusion criteria for this study were staged disease according to the American Joint Committee on Cancer criteria, no evidence of distant metastasis, surgically resectable tumor, chest CT scan with no evidence of metastasis or second primary, white blood cell count ≥3500/ mm3, platelet count ≥100,000/ mm3, creatinine clearance ≥60 mL/min3500/ mm3, bilirubin <1.5 mg%, and age ≥18 years. Patients with prior head and neck malignancy, history of head or neck irradiation, prior chemotherapy, medical contraindication to chemotherapy, surgery, or radiation, and positive pregnancy test were excluded. All patients were informed of the investigational nature of the study and signed a written consent for participation in accordance with institutional guidelines.
Our study group included 6 men and 3 women. Patient ages ranged from 49 to 72 years (average age, 56.3 years). The primary sites included tonsil (n = 2), base of tongue (n = 5), vallecula (n = 1), and glosso-tonsillar sulcus (n = 1). The tumors were staged according to the tumor node metastasis (TNM) system and included stage 3 (n = 2) and stage 4 (n = 7) lesions. Local staging included T2,2 T3,3 and T44 tumors. CTP studies were performed 1 day before endoscopy and biopsy under anesthesia. The mean interval between the baseline and follow-up studies was 26.5 days (range, 22–31 days).
Study Plan
For longitudinal prospective assessment, all patients in this study were examined with CT scans of the neck, CTP studies, and endoscopy under anesthesia before undergoing induction chemotherapy (baseline). Tumor margins were marked or tattooed during endoscopy by using an injected India ink suspension. Endoscopic tattooing is a means of permanently labeling a site by intramural injection of a pigment and facilitates site identification when viewed from the luminal surface. Imaging studies were performed 1 day before the endoscopic assessment. Induction chemotherapy comprised Cisplatin (100 mg/m2) on day 1 and 5-Fluorouracil (1000 mg/m2) daily for 5 days. All patients were enrolled for a follow-up imaging and endoscopic assessment, 3–4 weeks after the start of induction chemotherapy.
Patients with ≥50% reduction in tumor volume after induction chemotherapy (as measured on endoscopy) were classified as responders and received concomitant chemoirradiation therapy. Patients with <50% reduction in tumor volume were classified as nonresponders and underwent surgery and radiation therapy.
Blinding
The oncologist and the surgeon performing the endoscopy were blinded from the results of CT and the CTP. The radiologist evaluating the imaging studies was blinded from the results of endoscopy and biopsy.
Imaging Studies
All perfusion CT scans were obtained on a multidetector scanner (Lightspeed Ultra; General Electric Medical Systems, Milwaukee, Wisc). CT of the neck along with CTP was performed by using the following technique. A “localizer” noncontrast CT was obtained through the known primary site. The area of interest for measuring perfusion was centered on the largest area of gross anatomic abnormality identified on the noncontrast localizing images. Four adjacent 5-mm sections were selected at the level of the tumor. Fifty milliliters of nonionic contrast (300 mg/mL) were injected at 4 mL/s. At 5 seconds into the injection, a cine (continuous) acquisition was initiated by using the following parameters: 120 kV, 60 mA, 4 × 5 mm sections, 1 second rotation for duration of 50 seconds. The 1-second images were reformatted at 0.5-second intervals, and the 5-mm sections were reformatted into 10-mm-thick sections.
After completion of the perfusion acquisition, intravenous contrast was administered at 2 mL/s, and the routine neck study was obtained by using 2.5-mm contiguous sections (120 kV, 180 mA, 0.8-second rotation) performed from the skull base to the thoracic inlet. The perfusion data were postprocessed by using the commercially available Perfusion-2 software package on an Advantage Windows workstation (General Electric Medical Systems, Milwaukee, Wisc). A region of interest was placed in the internal carotid artery and internal jugular vein to generate contrast enhancement curves. The data were processed into maps that represented CP, BV, BF, and MTT. A single observer, who was aware of the primary tumor site, subsequently obtained regions of interest (25–30 mm2) through the primary tumor by using the following method. The tumor was localized on the contrast-enhanced CT by the radiologist. Specifically, the radiologist identified the level at which the tumor cross-section was largest. A user-defined region of interest was drawn freehand incorporating as much of the solid portions of the tumor as possible and omitting the necrotic regions. Care was taken to avoid encroaching on tumor boundaries to exclude peritumoral hyperemia.
Data and Statistical Analysis
For all statistical analyses, we initially calculated the difference between the posttreatment and the pretreatment CTP parameters (CP, BV, BF, and MTT). Treatment was defined as one cycle of induction chemotherapy.
We initially assumed that a reduction of ≥50% of tumor volume, as assessed by endoscopy, represented the standard of reference for tumor response to induction chemotherapy. To determine a statistically significant difference in CTP parameters between responders and nonresponders, the Wilcoxon rank sum test, a nonparametric test for nonpaired data, was employed. We ranked the absolute value of the difference between the posttreatment and pretreatment CTP parameters. Statistical significance was set at P < .05.
To account for the potential imperfection of the standard of reference as a true marker for response, we determined the agreement between endoscopic and CTP detection of response by using the kappa statistic.12,13 Comparison of agreement between endoscopic response as defined by a ≥50% reduction in tumor volume and response was defined in each of the CTP parameters as follows: (1) CTP response as ≥20% reduction in BF; (2) CTP response as ≥20% reduction in BV; (3) CTP response as ≥20% reduction in CP; and (4) CTP response as ≥20% prolongation of MTT, was performed to test the independent agreement of each parameter with the endoscopic assessment of response. We chose 20% as the threshold, on the basis of graphic representation of the distribution of CTP values. Strength of agreement was rated as poor (<0), slight (0–0.20), fair (0.21–0.4), moderate (0.41–0.6), substantial (0.61–0.8), or almost perfect (0.81–1.00).
Results
Endoscopic Response
The estimate of tumor volume reduction was quantified by visual assessment of tumor volume relative to area tattooed at the time of baseline endoscopy (Table 1). Seven of 9 patients showed ≥50% reduction in tumor volume after induction chemotherapy (responders). These patients subsequently underwent concurrent chemoirradiation therapy. Two patients failed to show adequate response to chemotherapy and were transferred to the surgery-radiation therapy arm (nonresponders).
Comparison of percentage change in CT perfusion parameters with endoscopic reduction in tumor volume
Changes in CTP Parameters following Induction Chemotherapy
The percent changes in perfusion parameters and endoscopic tumor volumes are presented in Table 1. The absolute values of CTP parameters (BF, BV, CP, and MTT), before and after therapy, are shown in Table 2. In general, variable degrees of reduction in BF, BV, and CP values were observed in responders after induction chemotherapy. In the responder group, the mean value of BF decreased from 114.2 mL/100 g/min (baseline) to 45.1 mL/100 g/min (postchemotherapy) and the mean BV decreased from 5.11 mL/100 g (baseline) to 3.1 mL/100 g on follow-up (Fig 1). In contrast, mean value of BF in the nonresponders increased from 56.9 mL/100 g /min to 75.9 mL/100 g/min (BF value in patient 6 decreased from 55.46 mL/100 g/min to 31.76 mL/100 g/min, whereas the BF value in patient 7 increased from 58.4 mL/100 g/min to 96.36 mL/100 g/min). Similarly, BV increased from a mean value of 2.7 mL/100 g to 4.71 mL/100 g (BV value in patient 6 decreased from 3.2 mL/100 g to 2.94 mL/100 g, whereas BV value in patient 7 increased from 2.23 mL/100 g/min to 3.54 mL/100 g).
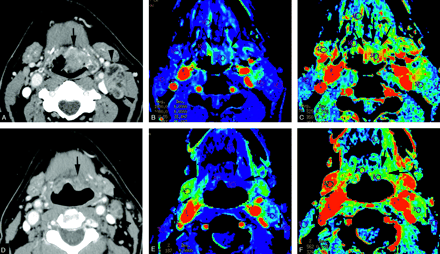
CTP in a patient with T2N2a carcinoma of the left valeculla before and after one cycle of neoadjuvant chemotherapy.
Axial CT image (A) through the level of the tumor, color maps of BF (B), and BV (C) were acquired before the start of therapy (baseline). Note a large tumor in the valeculla (arrows) before the start of therapy and a metastatic left cervical node (arrowhead). This tumor showed a high level of BF (131.5 mL/100 g/min) and BV (5.12 mL/100 g). Postinduction chemotherapy CT image through the level of the tumor (D), color maps of BF (E) and BV (F) at approximately similar anatomical level are also shown. A marked reduction in tumor size was noted after the chemotherapy (arrows). Also the perfusion maps demonstrate decrease in BF and BV. Quantitatively, there was 96% reduction in BF and 63% reduction in BV. Direct laryngoscopy showed nearly complete resolution of the tumor (patient 2).
Comparison of pretherapy and posttherapy values of BF (ml/100 g/min), BV (ml/100 g), CP (ml/100 g/min), and MTT (s)
The MTT showed more variable changes. Six of the 7 responders showed increased values of MTT on follow-up. In one responder (patient 1), MTT was reduced on follow-up examination. Among the nonresponders, one patient showed elevated (patient 6) and the other showed reduced value of MTT (patient 7) on follow-up.
Correlation of Endoscopic Response with CTP Parameters
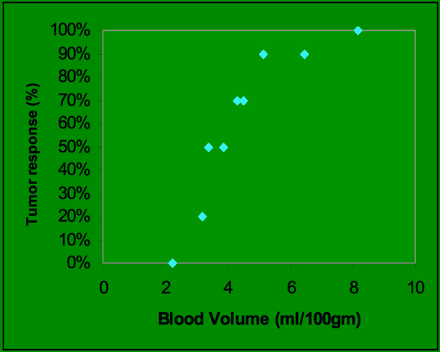
Responders had higher values of pretherapy BV compared with the nonresponders. There was a statistically significant correlation between higher values of BV and endoscopic tumor response (P < .05) [Fig 2]. Lower pretherapy levels of BV correlated with failed response to induction chemotherapy. Responders also had a trend toward higher levels of baseline BF compared with the nonresponders; however, correlation between BF measurements and endoscopic tumor response was not statistically significant (P = .14).
Graph showing the distribution of percent tumor response as a function of pretreatment BV. The pretherapy BVs ranged between 2.23 and 8.16 mL/100 g and endoscopic tumor response ranged between 0% and 100%.
There is substantial agreement between endoscopic assessment of response and ≥20% reduction in BV as a measure of response (kappa = 0.73). The agreements between endoscopy and reduction of BF and CP were fair (kappa = 0.37). Similarly, prolongation of MTT showed fair (kappa = 0.37) agreement with endoscopy.
Discussion
Tumor response to nonsurgical therapies is substantially influenced by tissue perfusion and local oxygen delivery.9–11,14 The oxygen supply to a tissue is governed by its perfusion and the arterial oxygen concentration.11 It is possible to measure tissue oxygen with the help of oxygen sensitive electrodes, but this technique is invasive and may not be suitable for assessment of many head and neck cancers because of their deep location.11 A noninvasive, quantitative method of assessment of tissue perfusion may provide a measure of tissue oxygenation and angiogenesis.8 In support of this hypothesis, a relationship between hypoxia and perfusion has been demonstrated in both animal and human tumors.15,16
A variety of approaches have been used for the radiologic assessment of tumor perfusion. These include contrast-enhanced dynamic CT,11 spin-labeling technique,9 blood oxygen level–dependent imaging with MR,17 and dynamic contrast-enhanced MR imaging.18,19 A CT-based method is advantageous, because CT is the most widely used diagnostic radiographic approach for assessing head and neck malignancies. Deconvolution-based CTP is a fast and robust imaging technique that is increasingly being used in the evaluation of intracranial vascular disorders.6 For a detailed discussion on the physical principles and mathematical considerations for deconvolution-based CTP technique, several excellent articles have been published.5,6,20,21
The physiologic basis of contrast enhancement closely matches that of tumor angiogenesis. Angiogenesis is associated with increased perfusion, BV, and permeability, all of which result in increased contrast enhancement. The degree of contrast enhancement is proportional to the intensity of neovascularization associated with the tumor. Therefore, CTP parameters such as BF, BV, CP, and MTT serve as surrogate measures for tumor angiogenesis.22
SCCAs of head and neck demonstrate significantly increased values of BF, BV, and CP and decreased values of MTT compared with normal structures.8 The elevated BF and BV in these tumors are likely due to tumor neovascularity, which has been demonstrated in several previous studies evaluating SCCA of the upper aerodigestive tract with angiography23,24 and histochemistry analysis of the tumor specimens.25,26 It is conceivable that higher levels of BF and BV may correlate with better oxygen/drug delivery and therefore may predict the response to radiation therapy or chemotherapy.
Measures of tumor perfusion assessed with dynamic CT or MR have been recently shown to be helpful in predicting response to radiation therapy.11,18 In a study by Hermans et al,11 the perfusion rate was shown to be an independent predictor of local outcome in patients who underwent radiation for the treatment of their cancer. To the best of our knowledge, this is the first report of the role of tissue perfusion in assessment of response to induction chemotherapy.
The induction chemotherapeutic regimen included 5-fluorouracil and cisplatin. Miller et al summarized the criteria for consideration as an anti-angiogenic agent as (1) in vitro cytotoxicity to endothelial cells at lower doses than required for cancer cells; (2) in vitro interference with endothelial cell function without cell death; (3) in vitro interference with specific steps of angiogenesis; and (4) in vivo evidence of antiangiogenic activity.27 In vitro studies of endothelial cell cultures demonstrated cisplatin’s dose-dependent increase in endothelial cell death even in the presence of vascular endothelial growth factor (VEGF), a proangiogenic protein expressed by a number of human tumors.28 Furthermore, a recent study by Lennernas et al evaluated elements of microvascular proliferation, microvessel segment length, and microvessel pattern formation to elucidate the antiangiogenic properties of cisplatin and fluorouracil. Although cisplatin and fluorouracil did not decrease VEGF-induced microvascular proliferation, these cytotoxins inhibited VEGF-induced changes in microvessel segment length and microvessel pattern formation.29 Antiangiogenic effects of fluorouracil and cisplatin may account for the measured changes in CTP parameters in responders.
In our study, CT determined pretherapy BV showed a significant correlation with endoscopic response. All the responders showed BVs ≥3.4 mL/100 g, whereas the nonresponders demonstrated BV levels of 3.2 and 2.23 mL/100 g, respectively. Reduction of BV on follow-up studies by ≥20% had a substantial correlation with ≥50% reduction in tumor volume compared with posttreatment alterations in other CP parameters. There appears to be more heterogeneity in pre- and posttreatment CTP parameters in nonresponders. In these 2 patients, variability of direction of CTP parameter change after induction chemotherapy likely reflects different pathologic processes. Furthermore, in at least one patient (6), BF values suggested response not confirmed on endoscopy, representing a false-positive. This has practical implications for the adoption of noninvasive CTP techniques in evaluating treatment response. Nevertheless, no test is perfect, and these preliminary results suggest that BV may represent a surrogate marker for tumor volume response to the induction chemotherapy. An association between tumor metabolism, tumor angiogenesis, and microvascular attenuation has been previously observed in cerebral gliomas.30 We hypothesize that a similar association between metabolic requirements of SCCA and BV may exist.
Although this was a prospective, nested trial, there are only enough subjects to draw preliminary conclusions, particularly in the nonresponse group. In light of the encouraging trends, further prospective studies in a larger patient population are warranted. We determined the tumor perfusion parameters from a single level by using a large region of interest. Therefore, these results may not be representative of entire tumor. Continued developments in the multisection technology and analysis software, however, may enable examination of entire tumor volume in the near future.11 We have used cervical internal carotid for the arterial input artery, whereas the branches of external carotid artery supply these tumors. The internal carotid artery was chosen because it was reliably identified on all scans in direct cross-sections. In addition, a previous study had demonstrated that there was no significant difference in BF, BV, MTT, or CP when the values were calculated by using either the ICA or ECA.31
In conclusion, a variety of clinical situations may benefit from quantitative assessment of tumor perfusion in the future. Potential applications include noninvasive in vivo assessment of tissue hypoxia during radiation therapy, measurement of response to therapies by using angiogenesis inhibitors and differentiation of recurrent/residual tumor from posttreatment changes.8 In particular, validation of the use of deconvolution CTP parameters may permit replacement of an invasive diagnostic procedure conducted under anesthesia currently used to assess tumor response with noninvasive perfusion-CT imaging.
References
- Received December 29, 2004.
- Accepted after revision June 10, 2005.
- Copyright © American Society of Neuroradiology