Abstract
PURPOSE: Intravenous methotrexate (IV-MTX), an effective treatment for acute lymphoblastic leukemia (ALL), has a significant toxic effect on the central nervous system, with leukoencephalopathy (LE) being the most common form. The purpose of this study was to use objective quantitative MR imaging to prospectively assess the temporal evolution of LE extent and intensity.
METHODS: Forty-five children (low-risk, 10 mol/L/12F; mean age, 5.0 years at diagnosis; standard/high-risk, 11 mol/L/12F; mean age, 9.2 years at diagnosis) treated for ALL on a single institutional protocol were evaluated longitudinally to assess the extent of LE (proportion of white matter impacted) through tissue segmentation and the relative intensity of LE through relative elevations in T1 and T2 relaxation rates. One-sided Wilcoxon-Mann-Whitney tests were used to assess differences in quantitative measures at 4 different points in therapy both within and between risk arms.
RESULTS: The proportion of white matter affected in both patient groups increased significantly with additional courses of IV-MTX, whereas the intensity of LE also increased steadily; however, both the intensity and extent of LE declined significantly ∼1.5 years after completion of IV-MTX. Increases in the T1 and T2 relaxation rates above normal-appearing white matter were significantly correlated with each other and were dependent on the proportion of white matter affected.
CONCLUSION: Higher doses and more courses of IV-MTX were associated with increased intensity and extent of LE. There was a significant reduction in both the intensity and extent of LE after completion of therapy. The impact of these changes on neurocognitive functioning and quality of life in survivors remains to be determined.
With improved treatment outcome in children with cancer, current emphasis is placed on the survivors’ quality of life, including neurocognitive function. Acute lymphoblastic leukemia (ALL) is the most common childhood cancer and affects 2,400 children annually in the United States. The 5-year event-free survival estimate for pediatric patients with ALL is ∼80% (1). Methotrexate given intravenously (IV-MTX) at high dose has been shown to decrease hematologic, testicular, and central nervous system (CNS) relapse. It has a significant toxic effect on the CNS, however, and can potentially lead to severe neurologic morbidity. Leukoencephalopathy (LE), seen as white matter hyperintensities on T2-weighted MR imaging, is the most common manifestation and may be either persistent or transient (2). The frequency and severity of LE may be dependent on the dose, cumulative exposure, and other clinical variables.
Most recent protocols containing IV-MTX routinely used MR imaging to evaluate neurotoxicity qualitatively. In those studies, the degree of LE was rated on a subjective grading scale. Many studies (3–5) used a variant of the original scale proposed by Wilson et al in 1991 (6). In these studies, normal examinations were rated as 0. Grade 1 corresponded to mild changes that did not extend to the gray-white matter junction (0%–25% of white matter involved). Moderate changes extending to the gray-white matter junction were rated as grade 2 (25%–50% of white matter involved). Grade 3 was reserved for severe changes involving the gray-white matter junction and were continuous throughout the centrum semiovale (>50% of white matter involved). Other studies classified patients only as normal, probable, or definitely abnormal, with no assessment of the extent of white matter involvement (7, 8). None of these studies evaluated intra- and interobserver variance. This subjective grading precluded comparison of therapy-induced LE between clinical trials and did not provide any continuous measure of the intensity or extent of LE as a function of other influential factors.
The present study builds on previous work (9) that examined the longitudinal incidence of LE in children treated for ALL on a single institutional protocol, which included 7 courses of IV-MTX and multiple intrathecal therapies with MTX, hydrocortisone, and cytarabine, but no cranial irradiation. The study found that higher doses and more courses of IV-MTX resulted in higher risk of developing LE; many of the changes resolved after completion of therapy. The present study used quantitative MR measures to longitudinally assess the extent of LE (proportion of white matter affected) through tissue segmentation and the relative intensity of LE through relative elevations in T1 and T2 relaxation rates. The purpose of this study was to prospectively and objectively assess the temporal evolution of LE extent and intensity in patients treated for ALL without cranial irradiation.
Methods
Patient Population
Consecutive patients at least 1 year of age enrolled on an institutional ALL treatment protocol between July 30, 1998, and August 30, 1999, were eligible for the study. Diagnosis of ALL was established by morphologic, cytochemical, immunophenotyping, and genetic studies. Patients must have received no more than 1 week of prior therapy with only glucocorticoids. Because this study was designed to assess the longitudinal incidence of LE in otherwise normal-appearing brain, patients with other neurologic complications, such as thrombosis, were not eligible. Written informed consent was obtained from the patient, parent, or guardian according to institutional review board, National Cancer Institute, and Office for Human Research Protections guidelines. Fifty-three patients were enrolled on the treatment protocol; however, 3 patients were <1 year of age, 3 died during induction, one elected to withdraw from the protocol, and one had thrombosis at presentation. These exclusion criteria resulted in 45 patients eligible for this study: 21 male patients and 24 female patients aged 1.5–18.6 years (mean, 7.1 years) at diagnosis (Table 1).
Demographic and descriptive statistics for patients imaged at each time categorized by therapeutic risk arm
Total number of patients evaluable at each time and proportion with leukoencephalopathy*
Treatment and Procedure Timeline
On the basis of a comprehensive risk classification—including blast cell immunophenotype and genotype, presenting clinical features, and early treatment response—patients were assigned to a risk group: low, standard, or high risk (corresponding to standard-, high-, and very high-risk categories in other protocols). B-cell precursor patients aged between 1 and 10 years and presenting with leukocyte count <50 × 109/L, leukemic cell DNA index = 1.16, or TEL-AML1 fusion were provisionally classified to have low-risk ALL, provided that they did not have testicular or CNS leukemia (ie, CNS3 status), hypodiploidy (<45 chromosomes), E2A-PBX1 fusion, or MLL rearrangement. Patients with BCR-ABL fusion (Philadelphia chromosome) were designated to have high-risk disease, and all others—including all T-cell ALL cases—were provisionally classified to have standard-risk ALL. Final risk status depended on the response to remission-induction therapy. Any patients with 0.01%–0.99% residual leukemia after completion of 6-week induction therapy were considered to have standard-risk ALL and received intensive postremission therapy, whereas those with 1% or more residual disease were designated to have high-risk ALL and were candidates for allogeneic hematopoietic stem cell transplantation.
All patients received 7 courses of IV-MTX, with dosage depending on the risk classification of individual patients (10). Patients with standard- or high-risk ALL received high-dose MTX at 5 g/m2 and those with low-risk ALL at 2.5 g/m2. Patients underwent MR examinations at 4 points during therapy: after one course (week 6 of remission induction), after 4 courses (week 7 of continuation therapy), after 7 courses (week 31 of continuation therapy), and at week 120 of continuation treatment. Week 120 of continuation therapy was the end of therapy for the female patients, but the male patients received an additional 26 weeks of continuation therapy. The timing of this last imaging examination was chosen to ensure that all patients were evaluated at approximately the same point in therapy.
Age-adjusted triple intrathecal chemotherapy with MTX, hydrocortisone, and cytarabine was given on days 5 and 26 of remission induction. Because of their increased risk of CNS relapse, additional intrathecal therapy was given on days 12 and 19 of remission induction in patients with CNS2, CNS3, traumatic lumbar puncture with blasts, T-cell ALL with leukocyte count >50 × 109/L, B-cell precursor ALL with leukocyte count >100 × 109/L, presence of Philadelphia chromosome, MLL rearrangement, or hypodiploidy <45 chromosomes. Triple intrathecal chemotherapy was given every week during consolidation therapy, and then on weeks 1, 2, 7, 12, 15, 23, 28, 31, 39, 47, and 54 of continuation therapy in low-risk cases and additional courses on weeks 19, 36, and 43 in standard-risk and high-risk cases; those at particularly high risk of CNS relapse (ie, T-cell ALL with leukocyte count >50 × 109/L, B cell precursor ALL with leukocyte count >100 × 109/L, presence of Philadelphia chromosome, MLL rearrangement or hypodiploidy <45, or CNS-3 status) received twice-weekly intrathecal therapy from weeks 54 to 56 of continuation therapy. Thus, the total number of intrathecal treatments ranged from 16 in low-risk cases with CNS-1 status to 25 in high-risk cases with CNS-2, CNS-3, or traumatic tap status.
MR Imaging
LE is best visualized with a T2-weighted sequence, preferably with CSF attenuated. The imaging protocol was designed to simultaneously yield raw images for the segmentation procedures and images necessary for the clinical evaluation of the patients. All MR imaging examinations were performed without contrast agent on a 1.5T Vision (Siemens Medical Systems, Iselin, NJ) whole-body imager with a standard circular polarized volume head coil. To minimize variability, a localizer sequence was used to determine the position of the patient in the coil. All images used in this study were acquired as 3-mm-thick contiguous oblique transverse-imaging sets defined by the most inferior extent of the genu and the splenium of the corpus callosum on the midline sagittal image. T1-weighted images were acquired with a multiecho inversion recovery imaging sequence (TR, 8000 ms; TE, 20 ms; TI, 300 ms; 7 echoes). proton density (PD)- and T2-weighted images were acquired simultaneously with a dual spin-echo sequence (TR/TE1/TE2 = 3500 ms/17 ms/102 ms). Fluid-attenuated inversion recovery (FLAIR) images were acquired with a multiecho inversion recovery sequence (TR/TE/TI = 9000 ms/119 ms/2470 ms; 7 echoes).
Although the complete imaging set was used in the segmented volumetric studies, only one section was used to determine quantitative relaxation measures. The section selected was at the level of the basal ganglia, included the genu and splenium of the corpus callosum and generally showed the putamen and the lateral ventricle. Quantitative T1 imaging was performed by using an adapted precise and accurate inversion-recovery (PAIR) sequence (11–14). The section was sampled with a single 3-mm-thick section acquired with a phase-sensitive, multiecho inversion-recovery sequence (TR, 2500 ms; TE, 29 ms; TI, 100, 500, 900, and 2389 ms; 7 echoes). Quantitative T2 imaging was performed with a series of spin-echo images at the same level. The section was sampled with a 3-mm-thick section acquired with a multiple spin-echo imaging sequence (TR = 2000 ms; 16 echoes evenly spaced from 22.5 to 360 ms; one acquisition).
Objective Detection of LE
Registration methods developed by Ostuni et al (15) were used to register all MR imaging sets within an individual examination. A correction for RF inhomogeneities in the imaging sets was performed with a modified renormalization transformation combined with a local contrast ratio measure for in-section correction and a polynomial modeling for correction across the sections (16). A combined imaging set consisting of T1, T2, PD, and FLAIR MR images and white matter, gray matter, and CSF a priori maps from a spatially normalized atlas were analyzed with a neural network segmentation based on a Kohonen Self-Organizing Map (17, 18). To improve the differentiation between true abnormal regions and CSF-contaminated regions with high FLAIR signal intensity (SI), an in-plane gradient magnitude threshold was added to the segmentation algorithm (19). After the segmentation was completed, the segmented maps were manually classified to identify the prototypical vectors associated with normal-appearing genu and diffuse white matter hyperintensities. The prototypical vector associated with the segmented region corresponding to areas of highest SI on T2 and FLAIR images was then compared with normal-appearing genu vectors from the same patient. This enabled each patient to act as their own control and adjusted for known maturational changes in the relaxation properties of white matter and acquisition variance caused by coil loading and tuning. This quantitative methodology has no observer variability and has been shown elsewhere to agree with radiologists’ reading of LE in 81% of cases (20).
Assessment of LE Extent
Quantitative MR imaging assessment of LE is difficult, because the MR properties significantly overlap those of normal tissue. We have elsewhere reported the use of an automated procedure for longitudinal measurement of tissue volume to quantify LE (18, 19). The procedure used a white matter mask combined with an automated, hybrid neural network to segment regions of LE and normal-appearing brain tissues. Five examinations from each of 5 volunteers (25 examinations) were used to test the reproducibility of baseline and subsequent, normal-appearing images; the coefficients of variation were <2% for gray and white matter. Regions of LE in patients were assessed by comparison with manual segmentation by 2 radiologists. Kappa analyses showed that the 2 observers’ interpretations agreed with the results of the automated procedure (κ = 0.70 and 0.74). Once each examination was segmented, LE and normal-appearing white matter (NAWM) volumes were assessed for all transverse sections from the apex of the brain to a level 6 mm below the corpus collusom. The proportion of white matter affected was defined as the total volume of LE over the combined volume of LE and NAWM.
Assessment of LE Intensity
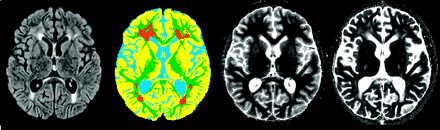
After acquisition, the 4 PAIR images and the 16 spin-echo images were used to generate quantitative T1 and T2 relaxation maps. T1 relaxation times were calculated by using a Levenberg-Marquardt algorithm to fit SI values (11). A parametric T1 map was produced on which pixel grayscale values were equivalent to the T1 relaxation time in milliseconds. As in the T1 analysis, T2 relaxation times were calculated by using a Levenberg-Marquardt algorithm to fit SI values to a monoexponential model. The curve-fitting procedure returned a T2 value for each pixel; this value was used to produce a parametric T2 map wherein pixel grayscale values were equivalent to the T2 relaxation time (in milliseconds). Error of the fit for both T1 and T2 maps was determined on a pixel-by-pixel basis, and further analysis excluded those pixels identified as having error above a statistical threshold (12). Segmented tissue maps of the corresponding section were used to automatically identify regions of LE and NAWM (Fig 1). Relative LE intensity was defined as the difference between the mean T1 or T2 of LE and NAWM for the same subject in the same section.
A single section from a typical examination after completion of IV-MTX demonstrating LE appearance on FLAIR image, segmented tissue map, quantitative T1 and quantitative T2 maps (left to right). LE is most evident in the frontal white matter.
Statistical Analyses
The current study builds on previous work, which examined the longitudinal incidence of LE in the same population. The study found higher doses and more courses of IV-MTX placed patients at a higher risk of developing LE; many of the changes resolved after completion of therapy. On the basis of these findings, the current measures of intensity and extent of LE were predicted to also increase with higher doses and more courses of IV-MTX. Patients were divided into 2 groups according to risk arm on the protocol (low risk and standard/high risk), which corresponded to 2 distinct targeted systemic doses of IV-MTX. A nonparametric one-sided Wilcoxon-Mann-Whitney test was used to test the differences in quantitative measures of extent and intensity at 4 different points in therapy both within and between risk arms. The reason for by using Wilcoxon-Mann-Whitney test was that some of the quantitative measures of extent and intensity at some time points were determined to be not (or not strongly) normally distributed with a Shapiro-Wilk test for normality. For consistency, nonparametric statistical methods were used throughout all analyses. Simple linear robust regression analyses were used to assess whether there was a significant correlation between any 2 of the 3 measures of extent and intensity of LE within a risk group. Data points with proportion white matter affected <0.1 were excluded.
Results
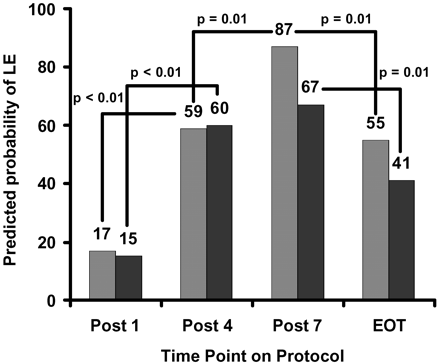
All patient groups, regardless of risk arm, demonstrated a pattern of LE intensity and extent similar to the longitudinal incidence pattern demonstrated previously (Fig 2). Not every patient was evaluated at every time point (Table 2). Prevalence of LE increased significantly after 4 courses of IV-MTX and the prevalence in the standard- and high-risk group increased further with an additional 3 courses of IV-MTX.
Predicted probability of developing LE according to a general linear model previously reported for patients on the standard- or high-risk arm (light gray bars) and patients on the low-risk arm (black bars) of the treatment protocol. Quantitative MR measures were evaluated post 1, 4, and 7 courses of IV-MTX and at end of therapy (EOT).
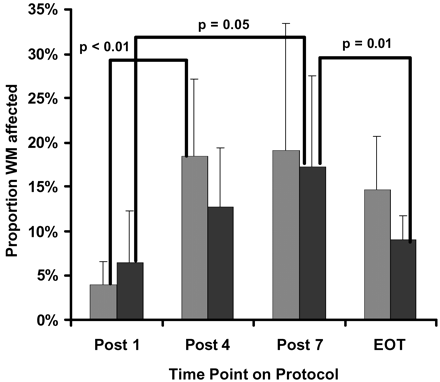
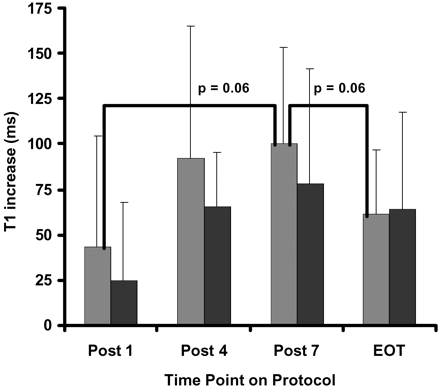
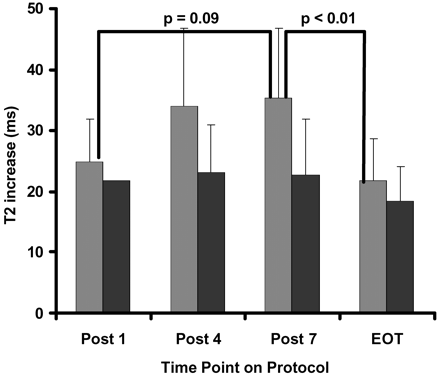
As was hypothesized, higher doses and more courses of IV-MTX were related to increased extent and intensity of LE. The proportion of white matter affected in the low-risk group rose steadily and became significantly different after completion of all 7 courses (Fig 3). Similarly, the proportion of white matter affected in the standard- and high-risk group increased significantly after 4 courses of IV-MTX and then remained approximately constant with additional courses. The intensity of LE changes measured by T1 and T2 relaxation rates (Figs 4 and 5, respectively) rose steadily with more courses of IV-MTX for both patient groups. These changes trended toward, but never reached, statistical significance between different time points. The standard- and high-risk groups, however, did exhibit significantly higher T2 elevations (P < .01) in LE at the completion of the IV-MTX compared with those patients treated on the low-risk arm.
Proportion of white matter affected (extent of LE) for patients on the standard- or high-risk arm (light gray bars) and patients on the low-risk arm (black bars) of the treatment protocol. Statistical results from a nonparameteric one-sided Wilcoxon-Mann-Whitney test are shown for both within and between groups at each of the 4 time points. Quantitative MR measures were evaluated post 1, 4, and 7 courses of IVMTX and at end of therapy (EOT).
Increase in T1 relaxation rate of LE over NAWM (intensity of LE) for patients on the standard- or high-risk arm (light gray bars) and patients on the low-risk arm (black bars) of the treatment protocol. Statistical results from a nonparameteric one-sided Wilcoxon-Mann-Whitney test are shown for both within and between groups at each of the 4 time points. Quantitative MR measures were evaluated post 1, 4, and 7 courses of IV-MTX and at end of therapy (EOT).
Increase in T2 relaxation rate of LE over NAWM (intensity of LE) for patients on the standard- or high-risk arm (light gray bars) and patients on the low-risk arm (black bars) of the treatment protocol. Statistical results from a nonparameteric one-sided Wilcoxon-Mann-Whitney test are shown for both within and between groups at each of the 4 time points. Quantitative MR measures were evaluated post 1, 4, and 7 courses of IV-MTX and end of therapy (EOT).
Between the completion of IV-MTX and the last time point near completion of therapy, the prevalence of LE has been shown to reduce significantly by almost a half for both patient groups. Similarly, both extent and intensity of LE decreased by the fourth time point. Although proportion of white matter affected did decrease for both patient groups, this reduction reached statistical significance only for the low-risk group. In addition, the standard- and high-risk group did exhibit significantly larger proportions of white matter affected (P = .02) compared with those patients treated on the low-risk arm. Reduction in T1 and T2 intensity also decreased for both patient groups during this time. In contrast to the extent measure, the reduction in T2 intensity of LE only reached statistical significance for the standard- and high-risk group.
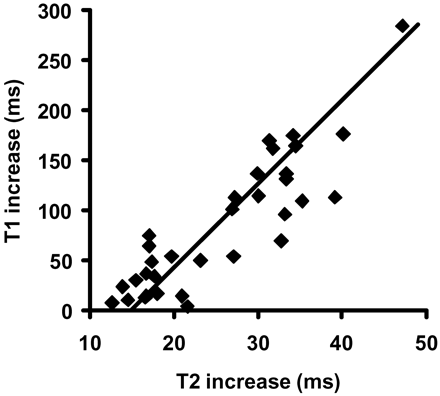
As the proportion of white matter affected increased, the T1 and T2 intensity also increased. The simple linear robust regression analyses revealed that, whereas the relative increases in T1 were not significantly dependent on increased extent, relative increases in T2 were significantly dependent (r = 0.42; P = .009). Further analysis also revealed a significant correlation (r = 0.77; P < .001) between relative increases in T1 and T2 (Fig 6). Measures of increased T1 and T2 in the same patients demonstrated an 8-fold larger increase in T1 compared with T2; however, normalizing the relative increase to the relaxation time of the NAWM yields an approximately equivalent percent increase for the 2 relaxation mechanisms. Approximately equivalent T1 and T2 elevations would be consistent with increased extracellular water due to an inflammatory response, though this does not rule out other mechanisms. These results at least support the acquisition of either quantitative T1 or T2 measures, but not necessarily both.
Scatter plot of increase in T1 and T2 relaxation rates for patients on both arms of the treatment protocol. The solid line is the result of a simple linear robust regression analysis. Increases in T1 and T2 relaxation relative to NAWM were highly correlated.
Discussion
This study was the first to use objective quantitative MR imaging measures to examine the intensity and extent of the longitudinal development of LE in 45 children treated for ALL on a single institutional protocol with CNS prophylaxis, including 7 courses of IV-MTX. Increasing exposure, corresponding to more courses and higher doses of IV-MTX, were each associated with increased intensity and extent of LE. Proportion of white matter affected in both patient groups increased significantly with additional courses of IV-MTX, whereas the intensity of LE also increased steadily; however, both the intensity and extent of LE reduced by approximately one-third between the completion of IV-MTX and the last time point for both patient groups. This finding, combined with previous work, suggested that some, but not all, LE was transient and may continue to decrease in prevalence, extent, and intensity with longer follow-up after completion of therapy.
Both IV- and IT-MTX are associated with demyelination, loss of oligodendroglial, and atrophy of the deep cerebral white matter. In a recent report of 5 subjects, acute MTX neurotoxicity was found to be closely associated with IT-MTX (21). Other reports have failed to show an association between IV-MTX and acute neurologic toxicity (22). These findings are consistent with our own experience with acute neurologic toxicity associated with IT-MTX and chronic neurotoxicity, such as decreased cognitive functioning more likely associated with IV-MTX and associated LE. A very recent study of children treated with chemotherapy alone found substantial adverse effects on intelligence and memory functioning with LE being observed on at least one MR imaging in 78% of subjects (23).
Historically, the association of LE and neurocognitive deficits has been indeterminate. Measurements of LE usually consisted of detection and sometimes subjective grading of extent. The developing brain may be more susceptible to damage due to newly synthesized myelin having a higher metabolic activity and lower stability making it more vulnerable to toxic effects of therapy (4). The location, extent, and intensity of LE may have a direct impact on the maturation of white matter in specific regions. If the normal maturation of white matter is disrupted by even transient LE, it may result in lower volumes of white matter with decreased integrity of the myelin compared with age-matched peers. Cognitive deficits may be related to the total volume of white matter with a possible region specific threshold effect that must be surpassed before deficits become evident (24).
As in any study, there are limitations to the results and conclusions of the current study. One complicating factor is not every patient was evaluable at every time point. This is particularly true of the T2 measures, which were not started until the protocol had been underway for 9 months; however, the number of subjects evaluable at each time point was sufficient to perform the analyses. Another weakness in this study was the use of a single imaging section to determine quantitative relaxation measures. The PAIR technique used to acquire the quantitative T1 data has been validated against 2 spectroscopic techniques with a mean difference of only 3.1% (25) and an excellent precision (reproducibility) exemplified by a 2% coefficient of variation. Unfortunately, increased precision and accuracy are gained at the expense of longer acquisition times, resulting in decreased coverage. The quantitative T2 measurements were acquired to match this coverage. The placement of this index section was not ideal, because the largest extent of LE is frequently in the centrum semiovale, resulting in a possible results bias due to small regions of interest at this level. An ongoing clinical trial is uniformly acquiring all quantitative MR measures in a larger group of patients. The results of this study will be prospectively evaluated in the ongoing study. Another limitation of this study is the limited timeframe covered by the MR examinations. Only a few of the patients were imaged after completion of therapy, and we therefore could not assess if the remaining LE will continue to resolve. Finally, whether these LE changes would affect the neurocognitive function and quality of life in survivors is still unknown.
Conclusions
In summary, this study was the first to objectively assess the temporal evolution of LE extent and intensity in patients treated for ALL without cranial irradiation by using objective quantitative MR measures. The current study reported an increase in the intensity and extent of LE with increasing exposure, corresponding to more courses and higher doses of IV-MTX. Some of the LE changes are transient as evidenced by a significant reduction in both the extent and intensity of LE ∼1.5 years after completion of IV-MTX. Although the magnitude of the results in this study may be specific to this treatment protocol, the transient longitudinal patterns are likely applicable to other studies. The impact of these changes on neurocognitive functioning and quality life in survivors remains to be determined.
Acknowledgments
We thank Rekha Karuppiah, for her efforts in processing and analysis of MR examinations, and Vickey Simmons, for scheduling the examinations.
Footnotes
This work was supported in part by R01-CA90246 and by Cancer Center Support (CORE) grant P30-CA21765 from the National Cancer Institute, by the American Cancer Society FM Kirby Clinical Research Professorship, and by the American Lebanese Syrian Associated Charities.
References
- Received December 10, 2004.
- Accepted after revision April 12, 2005.
- Copyright © American Society of Neuroradiology