Abstract
BACKGROUND AND PURPOSE: A new neurovascular microstent, the Cordis Enterprise stent, composed of nitinol, with a closed cell design, was specifically developed for the treatment of wide-necked intracranial cerebral aneurysms. The purpose of this study was to evaluate the safety, feasibility, and initial clinical results of using this device in patients.
METHODS: In clinical evaluation, five patients ranging in age from 54 to 71 years were electively treated. The smallest aneurysm measured 3.3 × 2.9 mm, and the largest aneurysm measured 10.6 × 8.5 mm (neck and height measurements).
RESULTS: All five cases (100%) were technically successful without complications. In each case, the stent was accurately placed in the desired location, immediately followed by coil embolization to the desired degree of occlusion with a satisfactory result. The poststent and coil-occlusion angiogram demonstrated excellent blood flow across the stent, with satisfactory positioning of the coils within the aneurysm in all cases (100%). No patient suffered any clinical or neurologic complications, and all were discharged 1–3 days postprocedure, in stable condition with no new neurologic deficits.
CONCLUSION: In early clinical studies, the Cordis Enterprise stent performed well. The stent was able to be well visualized, deployed easily, could be repositioned if needed, and was accurately placed without technical difficulties. The closed cell design allowed all coils to be placed within the aneurysm and remain outside the flow of the parent artery. No periprocedural complications were encountered.
The endovascular approach to treating intracranial aneurysms, both ruptured and unruptured, has been gaining increasing acceptance worldwide as an alternative to neurosurgical clipping for the past several years (1–5). There are however, some limitations to the types of aneurysms that can be safely and effectively treated by current endovascular coiling techniques. Wide-necked (>4 mm or dome-to-neck ratio <2) and fusiform aneurysms without a well-defined neck are more difficult to treat because of the inability to ensure that the coils, once deployed, will remain safely within the aneurysm sac and not obstruct blood flow in the normal parent artery (6). In these cases, the use of both balloon-expandable stents and more recently nitinol self-expanding stents, as well as the balloon-assisted technique, have been reported as adjuncts to treatment (7–16).
Balloon-expandable stents have been largely replaced by self-expanding stent technology, because of ease of use, better deliverability, and lower tendency for vessel rupture and damage to the artery during deployment (17–21). The first generation of nitinol self-expanding stents designed for intracranial aneurysm use was of an open cell design (22–26); however, a newer-generation nitinol stent system, the Cordis Enterprise stent (Cordis Neurovascular, Miami, FL), has recently been designed and developed specifically for intracranial cerebral aneurysm treatment of wide-necked aneurysms. The advantages of this stent system are that it can be introduced into a standard microcatheter after access is achieved and be partially deployed as much as 70% within the parent artery—and recaptured and redeployed if needed—and the closed cell design improves the ability of the coils to remain within the aneurysm and not protrude into the normal parent artery.
We report our initial clinical experience of using the Cordis Enterprise stent system in the elective treatment of patients who had either failed initial endovascular coiling with recanalization and in those aneurysms deemed to have a wide neck and to be suitable for both stent placement and coiling.
Patients and Techniques
Cordis Enterprise Stent System
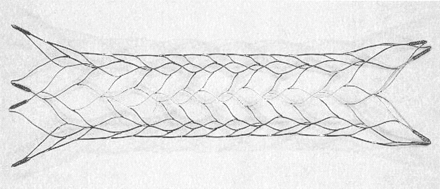
The Cordis Enterprise stent system was designed specifically for neurovascular applications to support embolic detachable platinum microcoils within wide-necked cerebral aneurysms. The stent has a closed cell design with flared ends (Fig 1). Each end has four radiopaque markers that flare out when fully deployed. The delivery system has an attenuated radiopaque marker band across the midportion of the stent to improve visualization during stent deployment across the aneurysm neck. The stent is compatible with a 0.021-inch Prowler Plus microcatheter (Cordis Neurovascular), which is initially placed beyond the aneurysm with any standard microguidewire.
Cordis Enterprise stent, a nitinol, self-expanding, microstent with a closed-cell design. When fully expanded, it is 4.5 mm in its central portion. The distal ends flare out, and each end has four radiopaque markers for enhanced visibility.
After the microcatheter is in place, the guidewire is removed and the stent is introduced into the hub of the microcatheter through an introducer sheath. The stent is then pushed through the microcatheter and placed across the aneurysm neck. The microcatheter is then carefully pulled back to unsheath the stent. As the stent is being deployed, up to 70% of the stent can be opened up, and stent placement verified by either direct fluoroscopy or angiography. If repositioning is required, the stent can be resheathed and the system either advanced forward or pulled back for more accurate placement. After the stent is fully deployed, the same microcatheter or, preferably, a second smaller microcatheter can then be used with a microguidewire to go through the interstices of the stent for coil occlusion of the cerebral aneurysm.
The initial stent system evaluated in clinical trials was 22 mm in length, expanded to 4.5 mm, and was designed to be used in blood vessels between 3.0 and 4.0 mm in diameter. Additional lengths and diameters of the stent are currently under development. The stent is highly flexible, easy to deploy, and well visualized.
Patients/Subjects
The Cordis Enterprise stent system was evaluated in five patients at our institution who were diagnosed with wide-necked intracranial cerebral aneurysms and who were electively admitted for the procedure. This was done under a U.S. Food and Drug Administration–approved clinical protocol with additional approval by the hospital investigational review board. Written consent was obtained from all patients. Patients were premedicated with aspirin 325 mg and clopidogril 75 mg, 24–72 hours before treatment (27–29). All patients underwent independent neurologic evaluation by a stroke neurologist before and after the procedure. Table 1 is a summary of the clinical information of the treated patients. There were three men and two women, ranging in age from 54 to 71 years. The aneurysm location included the posterior communicating artery (1), supraclinoid internal carotid artery (1), distal vertebral artery (1), and distal basilar artery (2). In four cases, the neck was >4 mm, and in all cases the aneurysm dome-to-neck ratio was <2. Three patients had prior subarachnoid hemorrhages related to their aneurysm. Three patients had initial coiling of their aneurysm and on follow-up angiography had significant recanalization requiring further treatment. One patient had a distal fusiform aneurysm without a well-defined neck and producing mass effect upon the brain stem with progressive neurologic symptoms. All procedures were performed under general anesthesia. A full three- or four-vessel cerebral angiogram was performed to evaluate the aneurysm fully, measure the aneurysm neck, width, and height, and measure the blood vessel proximal and distal to the aneurysm. Baseline activated clotting time (ACT) was measured in all patients, and all were given systemic anticoagulation with intravenous heparin, 70 U/kg, to achieve an ACT of 250–300 seconds, which was maintained throughout the procedure.
Characteristics of patients treated by stenting
A 6F Envoy guiding catheter (Cordis Neurovascular) was then guided into either the cervical internal carotid or vertebral artery, depending on the location of the aneurysm. A 2.3F Prowler Plus microcatheter with an internal diameter of 0.021 inches was then advanced over a 0.014-inch Agility microguidewire (Cordis Neurovascular) into the normal distal artery beyond the aneurysm by 2.5–3.0 cm. The guidewire was then removed, and the Cordis Enterprise stent was introduced into the hub of the catheter by using the delivery system.
Under fluoroscopic guidance, the Cordis Enterprise stent was then pushed through the microcatheter and aligned directly across the neck of the aneurysm. When proper alignment was achieved, the microcatheter was gently pulled back to unsheath the stent, while gentle forward tension was maintained on the stent system to keep it in place. The stent was deployed to as much as 70% of its opening, and positioning was confirmed by direct visualization or by performing cerebral angiography during partial deployment. If the stent required repositioning, it was resheathed, repositioned, and then redeployed.
Once the stent was fully deployed, the microcatheter and introducing system was removed. A smaller microcatheter was then reintroduced through the guiding catheter and, with direct fluoroscopic visualization, guided through the interstices of the stent and into the aneurysm. In all five cases, we used the Trufill DCS Orbit detachable coil system (Cordis Neurovascular) for occlusion of the aneurysm.
Following treatment, all patients were re-evaluated by a stroke neurologist and monitored in the neurologic intensive care unit for 24–72 hours. Patients were maintained on 325 mg aspirin and 75 mg clopidogril at least 4–6 weeks postprocedure.
Representative Cases
Case 2.
This 70-year-old man presented in July 2003 with a severe acute subarachnoid and intraparenchymal bleed, in Hunt and Hess grade V neurologic condition. He underwent emergent endovascular coiling of a large posterior communicating artery aneurysm and made a good neurologic recovery.
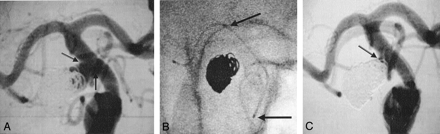
The follow-up angiogram demonstrated significant aneurysm recanalization, with the residual aneurysm neck measuring 5.5 mm, the height measuring 5.0 mm, and the width 4.9 mm (Fig 2A). Because of coil compaction and the wide neck of the residual aneurysm, it was decided to treat this with a combination of stent and coiling.
A, Posterior communicating artery aneurysm. Coil compaction with residual aneurysm filling. The neck width (arrows) is 5.5 mm and the dome height with residual aneurysm filling is 4.9 mm.
B, Enterprise stent (arrows) is placed across the neck of the aneurysm in the supraclinoid internal carotid artery.
C, Following stent placement and coiling, there is excellent blood flow across the stent and excellent coil occlusion of the aneurysm (arrow).
The Enterprise stent was placed across the wide neck of the aneurysm without difficulty in the supraclinoid internal carotid artery segment (Fig 2B). Following this, a Prowler 14 microcatheter (Cordis Neurovascular) was navigated through the interstices and four platinum Trufill DCS Orbit detachable coils were placed without difficulty. The poststent and coiling angiogram demonstrated an excellent result with no further filling of the aneurysm and good distal perfusion beyond the stent (Fig 2C). The patient was discharged home 2 days later in stable neurologic condition. At 30-days and 6-months of follow-up the patient continues to do well.
Case 4.
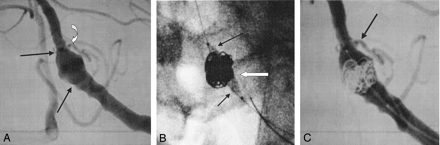
This 71-year-old man, presented with mass effect upon the brain stem from a large distal vertebral artery fusiform aneurysm. Cerebral angiography demonstrated a large fusiform aneurysm measuring 10.6 mm long, with a separate inflow and outflow, and 8.5 mm wide. In addition, the upper portion of the aneurysm incorporated the posterior inferior cerebellar artery (Fig 3A). The Enterprise stent was deployed without difficulty across the aneurysm neck. To ensure that no coils would protrude into the parent vessel, a 15-mm balloon was placed across the stent, and inflated, while eight platinum Trufill DCS Orbit detachable coils were deployed into the aneurysm (Fig 3B).
A, Fusiform aneurysm of the distal vertebral artery (arrows) measuring 10.6 mm in length, with the posterior inferior cerebellar artery arising from the distal portion of the aneurysm (curved arrow).
B, Enterprise stent has been placed across the aneurysm, a microcatheter (white arrow) is in the aneurysm, and a balloon (black arrows) is placed across the stent to ensure that the coils remain within the aneurysm and outside the parent artery.
C, A total of eight Orbit coils were placed into the aneurysm, with occlusion of the lower two-thirds of the aneurysm and preservation of the upper one-third to preserve blood flow to the posterior inferior cerebellar artery (arrow).
The coils were deployed to occlude the aneurysm circumferentially in the lower two-thirds region and not block the distal aneurysm segment, to preserve blood flow to the posterior inferior cerebellar artery. Temporary balloon inflation within the vertebral artery ensured that the coils would not encroach upon the normal artery during coil delivery. The poststent and coil-occlusion angiogram demonstrated excellent blood flow through the stent, excellent filling of the posterior inferior cerebellar artery, and occlusion of the lower two-thirds of the aneurysm (Fig 3C). The patient was discharged home 2 days later in stable condition and continues to do well at 30 days and 6 months of additional follow-up.
Case 5.
This 54-year-old woman presented in 1994 with symptoms due to mass effect from a giant, unruptured distal basilar artery aneurysm. She presented with chronic headaches, diplopia, slurred speech, and difficulty with gait. She had undergone several prior endovascular coil embolization treatments for her giant basilar aneurysm, and re-presented with coil compaction and significant residual aneurysm filling.
Cerebral angiography demonstrated that the basilar aneurysm had incorporated both the right and left superior cerebellar arteries and the right posterior cerebral artery into its base (Fig 4A). The left posterior cerebral artery was occluded. The residual aneurysm neck measured 6.9 mm, the height measured 7.9 mm, and maximum width measured 12.1 mm. To maintain blood flow in all three normal vessels arising from the base of the aneurysm, it was decided to place the distal flared end of the Enterprise stent directly into the aneurysm, 5 mm distal to the origin of both the right posterior cerebral artery and the superior cerebellar arteries, and then to coil above the stent within the aneurysm (Fig 4B). By simply placing the stent into the right posterior cerebral artery, we could not ensure that we would be able to protect the left superior cerebellar artery from occlusion.
A, A 54-year-old woman presenting with recurrence of a giant distal basilar artery aneurysm. The base of the aneurysm incorporates both superior cerebellar arteries (small arrows) and the right posterior cerebral artery (large arrow). The left posterior cerebral artery is occluded.
B, To maintain patency of both superior cerebellar arteries and the posterior cerebral artery, it was decided to place the stent 5 mm above the origin of the right posterior cerebral artery, directly within the aneurysm. The “white line” indicates stent deployment.
C, Following stent deployment, a total of 13 additional Orbit coils were placed above the stent and were well maintained in position by the deployed stent. The postocclusion angiogram demonstrates significant reduction in flow to the aneurysm, while maintaining sufficient blood flow to both superior cerebellar arteries (small arrows) and the posterior cerebral artery (large arrow) by the stent.
During the initial stent placement within the aneurysm, the stent shifted down by 3 mm, and, therefore, the stent was resheathed, readvanced 5 mm, and redeployed without difficulty. A total of 13 Trufill DCS Orbit detachable coils were then placed into the basilar artery aneurysm, above the stent, and the posttreatment angiogram demonstrated excellent patency of both superior cerebellar and the right posterior cerebral arteries, which were held in place by the stent (Fig 4C). The patient made an excellent recovery and was discharged home in stable condition. She remains well at 6 months of follow-up.
Results
In all five cases (100%), the Cordis Enterprise stent system was delivered and deployed to the intended location without difficulty. In four cases, the stent was placed across the aneurysm neck as intended, and in one case of a large basilar artery aneurysm in which there was incorporation of both superior cerebellar arteries and the posterior cerebral artery, we deliberately decided to place the top of the stent directly into the aneurysm and allow the struts to flare out across the neck of the normal blood vessels (case 5). In this case, there was a slight downward shifting of the stent, and therefore the stent was resheathed, repositioned 5 mm higher, and redeployed without difficulty.
In all five cases (100%), the microcatheter could be placed through or across the stent without difficulty and coil occlusion was performed by using the Trufill DCS Orbit detachable coil system, to provide the desired aneurysm occlusion. The poststent and postcoil angiogram demonstrated excellent blood flow across the stented arteries in all cases (100%), no evidence of distal branch occlusions, and excellent angiographic occlusion of the aneurysms. In case 4, which was a fusiform aneurysm of the distal vertebral artery, the upper portion of the aneurysm incorporated the posterior inferior cerebellar artery and we intentionally did not coil occlude this portion to preserve blood flow to this artery. In case 5, we intentionally did not occlude the base of the basilar aneurysm to preserve blood flow to both superior cerebellar and the posterior cerebral arteries.
All five patients (100%), remained neurologically intact and stable following the procedure, without any clinical sequelae related to the treatment. All patients were discharged home in stable condition within 48–72 hours after the procedure. All patients continue to do well at 6 months of follow-up.
Discussion
Endovascular and surgical treatment of wide-necked and fusiform intracranial cerebral aneurysms has remained technically challenging. Previous literature on endovascular coiling indicates that aneurysms with wide necks (>4 mm and/or dome-to-neck ratio <2) and larger aneurysms (>10 mm) tend to have lower rates of angiographic occlusion and higher rates of incomplete occlusion or aneurysm recanalization on mid- (6–12 months) and late-term (>1 year) follow-up angiography (30–37). In many instances, the treatment has been parent artery sacrifice, for very wide-necked and fusiform aneurysms (37). It is in these cases that intravascular stents may improve the endovascular technique for therapy.
The earliest clinical report of stent-assisted coiling of an intracranial ruptured cerebral aneurysm was by Higashida et al, in 1997 (38). Since then, with improvements in small-vessel stent technology, more reports from various centers reported good results in small series of cases. In 2000, approval was obtained in Europe for a balloon-expandable neurovascular stent (AVE/Medtronics, Santa Rosa, CA), developed specifically to treat wide-necked cerebral aneurysms. In 2002, the Neuroform stent (Boston Scientific Neurovascular, Fremont, CA) was approved in Europe, followed by US FDA approval, with a humanitarian device exemption (HDE) status, which allows as many as 4000 patients per year to be treated with this device. The Neuroform stent was the first self-expanding, nitinol stent, delivered through a microcatheter, with an open cell design, approved for use to treat wide-necked cerebral aneurysms (22–26). Some of the technical difficulties described with this stent design, however, included difficulty in stent delivery through the microcatheter particularly in tortuous bends, misdeployment of the stent if it was either too distal or too proximal to the aneurysm, stent migration during microcatheter placement into the aneurysm, stent herniation into the aneurysm, and, in some cases, the inability of the stent to adequately support the coil mass within the aneurysm due to the open cell design of the stent system. Several of these technical problems have been improved with newer delivery microcatheter systems, and fusing some of the open struts on the stent, to allow less of the stent to open up in larger wide-necked aneurysms.
Advantages of Closed Cell Design
The Cordis Enterprise stent system has overcome these previous technical difficulties. The Enterprise stent is a closed cell design, and therefore there is significantly less tendency for coils to protrude or herniate through the stent surface. The closed cell design allows the stent to be partially deployed up to 70% in the parent artery and to be reconstrained and recaptured if the initial deployment is not precise. This is not technically feasible with an open cell design. The stent is compatible with any standard braided microcatheter with an internal diameter of 0.021 inches, which maintains a uniform inner lumen diameter around curves.
In early clinical experience and animal and bench top testing, the Enterprise stent system performed exceptionally well in terms of ease of deployment, visibility at the ends, ability to negotiate tortuous bends, and stability upon deployment. No major technical problems with microcatheter access through the stent interstices or with detachable coil deployment were encountered in any of the clinical cases. There were no problems with any coils herniating through the stent interstices.
Potential Issues
Potential problems still need to be evaluated with any implantable device. These include the possibility of acute or delayed stent thrombosis, stent occlusion, thromboembolic problems, stent migration, in-stent stenosis due to intimal hyperplasia, and mid- and long-term results of aneurysm occlusion. In addition, there is a potential higher risk of treating patients with an intracranial stent with an acutely ruptured aneurysm, because of the need for dual antiplatelet therapy before treatment, which should be weighed against the potential benefits (39, 40).
We believe that these additional techniques and devices will continue to expand the ability of the interventional neuroradiologist and endovascular neurosurgeon to treat a wider variety of intracranial cerebral aneurysms with better results. It is possible that, as microstent technology continues to improve, more types of aneurysms may be better treated, with less incidence of recanalization, by combining both stents and coils to better occlude the aneurysm initially; however, prudent judgment is still required for patient selection, because long-term results are still pending. Therefore, these stents should in particular be used to treat those aneurysms that previously were determined to be difficult to treat with detachable coil-occlusion therapy alone.
Conclusion
The Cordis Enterprise stent is a new nitinol, self-expanding stent designed specifically to treat wide-necked intracranial cerebral aneurysms. In early clinical studies, the Cordis Enterprise stent performed well. The stent was able to be well visualized, deployed easily, could be repositioned if needed, and was accurately placed without technical difficulties. The closed cell design allowed all coils to be placed within the aneurysm, and remain outside the flow of the parent artery. No periprocedural complications were encountered.
References
- Received October 5, 2004.
- Accepted after revision December 27, 2004.
- Copyright © American Society of Neuroradiology