Abstract
BACKGROUND AND PURPOSE: In some patients with stenosis of an intracranial artery, navigating the balloon or stent-delivery system is difficult of tortuous anatomy of the aortic arch, carotid arteries, or vertebral arteries Our purpose was to describe techniques of intracranial stent placement that help in navigating the stent-delivery system in tortuous vessels.
METHODS: Between May 1998 and June 2004, 73 symptomatic stenotic (>50%) intracranial arteries were successfully treated with stent-assisted angioplasty. In 11 cases, standard techniques of navigating the stent-delivery system into the intended lesion failed because of vascular tortuosity. In these difficult cases, several techniques were used to overcome the tortuosity. Five lesions were located in the middle cerebral arteries, four were in the supraclinoid internal carotid arteries, and two were in the distal vertebral arteries.
RESULTS: In all difficult cases, stents were successfully placed in the intracranial artery by using several techniques: 1) waiting method in which we waited for 20–30 minutes after advancement of the microwire across the lesion, 2) the double-wire technique, and 3) the coaxial double–guiding catheter technique. The waiting method made smooth stent navigation possible in five cases, the double-wire technique was successful in four cases, and the coaxial double–guiding catheter technique was effective in two cases. No technique-related complications occurred.
CONCLUSION: In difficult cases in which standard techniques of navigating the stent-delivery system into the intended lesion fail because of vascular tortuosity, our techniques were useful methods for intracranial stent navigation.
Atherosclerotic stenosis of major intracranial arteries is an important cause of ischemic stroke and accounts for 8–52% of all cases of cerebral atherosclerosis (1–3). Atherosclerosis may selectively develop in intracranial vessels, particularly in African American and Asian individuals (2, 3). Traditionally, antiplatelet or anticoagulant therapy has been used as the first treatment technique, but it causes a notable number of strokes and deaths (4, 5).
Percutaneous balloon angioplasty has attracted attention in recent years as an alternative technique for treating critical intracranial stenosis (6, 7). In addition, elective stent placement for intracranial atherosclerotic stenosis has become an alternative because of the newer, more flexible coronary stents (8–11). To advance a balloon or stent-delivery system in the intracranial vessels, the tip of the guidewire should be placed as distally as possible and the guiding catheter should be positioned as close to the base of the skull as possible for good support. However, in some patients, navigating the balloon or stent-delivery system is difficult of tortuous anatomy of the aortic arch, carotid arteries, or vertebral arteries (9–12).
The purpose of this study was to evaluate several techniques of intracranial stent placement to help in navigating the stent-delivery system in patients with tortuous vessels.
Methods
Patient Selection
Between May 1998 and June 2004, 65 patients with 73 cases of symptomatic and significant intracranial atherosclerosis underwent stent-assisted angioplasty after giving informed consent. The lesions were located in 27 internal carotid arteries (ICAs), 27 vertebrobasilar arteries (VAs), and 19 middle cerebral arteries (MCA). Using the standard technique, we were able to navigate the stent-delivery system into the intracranial lesion in 62 patients. We included 11 patients in whom the standard technique to navigate the system into the intended lesion had already failed because of vascular tortuosity. Five lesions were in the MCA, four were in the supraclinoid ICA, and two were in the distal VA. This study population included seven women and four men with a mean age of 64.2 years (range, 42–77 years). The mean degree of stenosis was 71.8%. In these difficult cases, several techniques were used to overcome the vascular tortuosity. We retrospectively analyzed the feasibility and usefulness of these techniques for intracranial stent navigation in tortuous carotid or vertebral arteries.
Stent placement was recommended to decrease the risk of new or recurrent cerebral infarction in patients with significant atherosclerotic stenosis 1) when transient ischemic attacks or infarction recurred or progressed despite optimal medical therapy; 2) when anticoagulation or antiplatelet agents were contraindicated; 3) when patients had previous ischemic events with decreased cerebral perfusion, as shown on single photon emission CT before and after the intravenous administration of 1 g acetazolamide (Zoladin; Far-East Pharmaceuticals, Seoul, Korea) administration; or 4) when coronary artery bypass grafting was planned.
Significant stenosis was defined as stenosis of more than 50%, as estimated on digital subtraction angiography (Multistar; Siemens, Erlangen, Germany) according to the North American Symptomatic Carotid Endarterectomy Trial criteria. Symptomatic stenosis was defined as the occurrence of one or more transient ischemic attacks and/or nondisabling strokes in the territories of the stenotic artery within 6 months.
Standard Technique for Intracranial Stent Placement
Most of our study patients received premedication with aspirin 100 mg and clopidogrel 75 mg (Plavix; Sanofi-Synthelabo, Seoul, Korea) daily for at least 3 days before the procedure. In addition, low-molecular-weight nadroparin calcium 2850 IU/0.3 mL (Fraxiparine; Sanofi-Synthelabo) was injected subcutaneously two or three times a day during the same period.
In all patients, the therapeutic procedures were performed during a second angiographic session. The patient was fully awake during the procedure. ECGs, arterial oxygen saturation, and blood pressure were appropriately monitored. A unilateral intra-arterial approach was used after a standard Seldinger puncture and a 6F–7F introducer sheath was placed in the right femoral artery. The patient’s baseline activated clotting time (ACT) was obtained before the procedure. Patients then received systemic heparinization and a bolus injection of heparin 3000–5000 IU just before the therapeutic. A bolus of heparin 1000 IU was administered every hour to provide an ACT of longer than 250 seconds or twice the baseline ACT throughout the procedure.
A 6F–7F guiding catheter (Envoy; Cordis Endovascular Corporation, Miami, FL) was positioned in the distal cervical ICA or VA. The stenotic segment of the intracranial artery was then crossed with a 205-cm-long, 0.014-in. microwire (Transend, Boston Scientific Corporation, Miami, FL) as distally as possible to ensure maximal support while allowing tracking of the balloon-mounted stent catheter. The balloon-expandable coronary stent (S660 or S670, Medtronic, Minneapolis, MN; Flexmaster, Jo Med GmbH, Rangendingen, Germany) was gently advanced over the microwire and positioned across the stenosis by using the roadmapping imaging and external markings of the stent. The correct stent position was angiographically confirmed. We then began to deploy the stent by inflating the balloon. Balloon pressures did not exceed the burst pressure of the stent and balloon. When no gap between was observed between the stent and the parent artery, we ended stent deployment and waited for 1 hour to identify possible complications, including acute in-stent thrombosis or undetected vessel rupture. If final angiography showed no complications, we completed the procedure after measuring the rate of residual stenosis on the angiogram. After the procedure, hemostasis of the femoral artery was achieved by using an occlusion device (Angioseal; St Jude Medical, Minnetonka, MN).
Techniques to Overcome Vascular Tortuosity
The techniques were the waiting method, the double-wire technique, and the coaxial double–guiding catheter technique.
Waiting Method.—
When the standard technique to navigate the stent-delivery system into the intended lesion failed because of acute angles of tortuous vessels, it was stopped, and the system was withdrawn, leaving the microwire for delivery across the intracranial lesion. We then waited for about 20–30 minutes. If the microwire showed an obtuse angle after the waiting method, the stent-delivery system was renavigated.
Double-Wire Technique.—
If the stent-delivery system was not advanced and if angiograms showed persistent vascular tortuosity despite waiting, a second microwire was inserted across the acute angle. The tip of the second microwire was positioned distal to the tortuous segment. Then, the stent-delivery system was advanced again. When the system could not access the intended lesion, a 0.035-in., hydrophilic, angled guidewire (Radiofocus guidewire; Terumo Corporation, Tokyo, Japan) was inserted after the second microwire was withdrawn, and it was positioned proximal to the stenotic segment and/or distal to the acute angle. We then tried again to advance the stent-delivery system.
Coaxial Double–Guiding Catheter Technique.—
If the stent-delivery system could not reach the stenosis because of vascular tortuosity, we removed all devices, including the wires, the stent-delivery system, and the guiding catheter. An 8F guiding catheter was positioned in the distal cervical ICA or VA, and a 5F guiding catheter was coaxially inserted in the primary guiding catheter. We tried again to advance the stent-delivery system by using the aforementioned techniques. When the guiding catheter was pushed back from its position and when the stent-delivery system could not be advanced any more during the stent navigation despite the use of this technique, we did not continue the procedure.
Analysis of Technical Success and Complications
Technical success of these techniques was defined as reaching the target lesion with the stent-delivery system. Complications of the techniques were defined as complications observed during navigation of the stent into the target lesions.
Results
The Table summarizes the11 cases of intracranial stent placement by using our techniques. In all 11, navigation of the stent-delivery system into the target lesions was successfully performed by using several techniques. No vascular injuries (eg, vasospasm, dissection) occurred during or after stent navigation with the standard technique. Using the waiting method (Fig 1) alone, we successfully navigated five stents into the target lesions. In four cases, navigating the stent with the waiting method failed, but stents reached the target lesions by using the double-wire technique (Fig 2). In two cases in which these techniques failed, stents were successfully placed in the target lesion by using the coaxial double–guiding catheter technique (Fig 3).
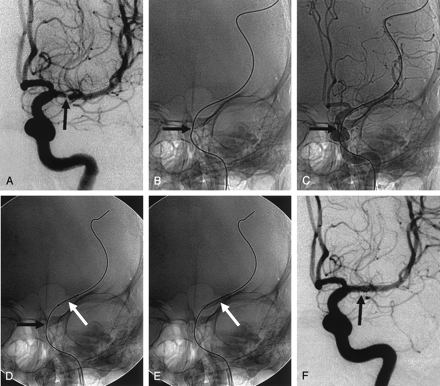
Waiting method. A, Anteroposterior left ICA angiogram shows >60% stenosis (arrow) of the M1 portion of the MCA.
B and C, Standard technique for stent navigation failed (not shown). Anteroposterior views obtained after the stent-delivery system was withdrawn show the angled microwire in the cavernous ICA (arrows).
D, After 20 minutes of waiting, anteroposterior view shows that the angle of microwire changed (black arrow), and navigation of the delivery system (white arrow) into the target lesion is successful.
E, Balloon-mounted coronary stent is successfully deployed (arrow).
F, Anteroposterior left ICA angiogram after stent placement reveals sufficient and smooth dilatation of the stenotic segment (arrow).
Double-wire technique.
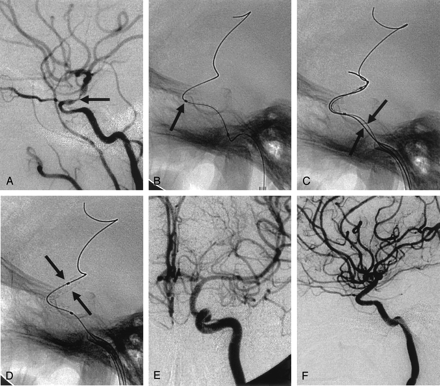
A, Lateral left ICA angiogram shows >70% stenosis (arrow) of the supraclinoid portion.
B, Lateral magnified view shows that the balloon-mounted stent cannot cross the acute angle (arrow) of the cavernous ICA.
C, Navigation of the stent-delivery system into the target lesion (arrows) is successful.
D, Balloon-mounted coronary stent (arrows) is successfully deployed. No procedure-related complication occurred.
E and F, Anteroposterior (E) and lateral (F) left ICA arteriograms obtained immediately after stent placement show sufficient and smooth dilatation of the stenotic segment.
Coaxial double–guiding catheter technique.
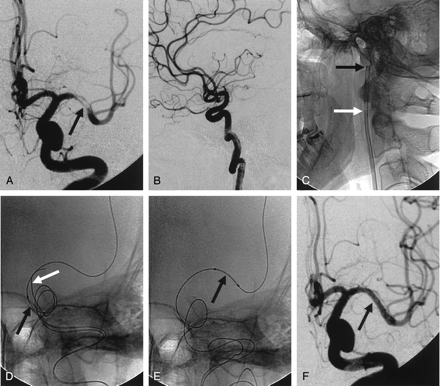
A, Anteroposterior left ICA angiogram shows >80% stenosis (arrow) of the M1 portion of the MCA.
B, Lateral left ICA angiogram shows marked tortuosity of the ICA.
C, After the standard, waiting, and double-wire techniques fail, an 8F guiding catheter (black arrow) is positioned in the proximal cervical ICA, and a 5F guiding catheter (white arrow) is coaxially inserted within it.
D, Anteroposterior magnified view shows that the balloon-mounted stent cannot cross the acute angle (black arrow) of the cavernous ICA. Second wire (white arrow) is inserted across the curve.
E, Navigation of the stent-delivery system into the target lesions is successful with the coaxial double–guiding catheter and double-wire techniques.
F, Anteroposterior left ICA angiogram obatined immediately after stent placement shows sufficient and smooth dilatation of the stenotic segment.
Of five MCA stents, two reached the target lesions by using the waiting method; one, by using the double-wire technique; and two, by using the coaxial double–guiding catheter technique. Of four ICA stents, two reached the target lesion by using the waiting method, and two, by using the double-wire technique. Of two distal VA stents, one reached the target lesion by using the waiting method; and one, by using the double-wire technique. No technique-related complications occurred during navigation of the stent into the target lesion.
Discussion
Vascular tortuosity is a major cause of difficulty or failure in treating intracranial lesions. In numerous reports on intracranial stent placement (9–11), vascular tortuosity is the most common cause of treatment failure in patients in whom stent placement for intracranial stenosis is attempted. In a report by Mori et al (9), two (17%) of 12 intracranial stent placements were not successful because of an inability to access the site of stenosis through a tortuous vessel. Our failure rate with the standard technique was 15.1%, which compares well with published results.
To advance a stent-delivery system in an intracranial vessel, the tip of the guidewire should be placed as distally as possible and the guiding catheter should be positioned as close to the base of the skull as possible for good support. The delivery system should be not forced; instead, it should be gently navigated into the intracranial lesion. Friction between the distal edge of the stent and the arterial wall prevents smooth navigation of stent-delivery systems, and forceful pushing may lead to serious complications such as dissection along the edge of the stent or deformation or migration of the stent. The former can cause pseudoaneurysm, which may lead to hemorrhage or thromboembolism. The latter may lead to abrupt closure of the intracranial artery and massive cerebral infarction. Therefore, a technique for safe and smooth intracranial stent navigation is required to perform this endovascular procedure without complications (13).
In this study, the standard stent technique to access the intended lesion failed because of vascular tortuosity in some patients. Therefore, we performed three techniques to help navigate a stent-delivery system. The first was waiting method, or simply waiting without any other manipulation for vascular tortuosity to resolve because of the stiffness of the microwire. This method is simple and effective. In our study, five cases were successfully treated in this way, without complications.
If the stent-delivery system could not be advanced and if angiograms showed persistent vascular tortuosity despite waiting, we applied the double-wire technique, which was introduced in other reports (13, 14). In this technique, a second wire is placed parallel and adjacent to the stent-delivery system. The adjacent wire appears to facilitate the advancement of the system in acutely angled vessels. The adjacent wire may diminish friction between the arterial wall and the delivery system. Another consideration is that the wire makes an obtuse angle to advance the stent-delivery system. We succeeded in four cases by using this method.
When these techniques failed, we applied the coaxial double–guiding catheter technique. In this method, another small guiding catheter is inserted into the primary guiding catheter. The stenotic segment of the intracranial artery was then crossed with a microwire as distally as possible to ensure maximal support. The stent-delivery system is gently navigated again. The role of the primary guiding catheter is to provide additional support for the second guiding catheter, preventing the second catheter from being pushed back from its position during advancement of the stent-delivery system. We succeeded in two cases by using this technique. When the guiding catheter was pushed back from its position (insufficient back-up support) and when the stent-delivery system could not be advanced any more during the stent navigation despite the use of this technique, we did not continue the procedure because aggressive intracranial stent navigation can lead to catastrophic complications, such as vascular dissection or stent migration.
A major disadvantage of our techniques is that they require additional, complex manipulation. A complex procedure may increase the risk of thromboembolic complications compared with the standard technique. Therefore, we believe that strict anticoagulation therapy is needed to prevent thromboembolism during the procedure. In addition, the relatively simple standard technique should be tried first. In our study, all procedures were gently performed while we monitored the angiogram at each step. No complications (eg, thromboembolism, vascular dissection) occurred in relation to our techniques.
Conclusion
Despite advances in stent technology, stent placement for intracranial atherosclerosis is still a challenging procedure. An aggressive approach for intracranial stent navigation may lead to catastrophic complications. In difficult cases in which standard techniques to place the stent-delivery system into the intended lesion failed because of vascular tortuosity, the aforementioned techniques for intracranial stent navigation may be feasible and useful.
Acknowledgment
We would like to thank Bonnie Hami, MA, Department of Radiology, University Hospitals of Cleveland, OH, for her editorial assistance in the preparation of this manuscript.
Footnotes
Presented in part at the European Congress of Radiology, Vienna, Austria, March 5–9, 2004, and at the 5th World Stroke Congress, Vancouver, British Columbia, Canada, June 23–26, 2004.
References
- Received July 23, 2004.
- Accepted after revision November 15, 2004.
Summary of 11 patients receiving intracranial stents
- Copyright © American Society of Neuroradiology