Abstract
Summary: A 43-year-old woman with two incidental paraclinoid internal carotid artery aneurysms underwent coil embolization of the larger superior hypophyseal aneurysm and 10 weeks later underwent follow-up angiography that showed regression of the smaller, more distal paraclinoid aneurysm. We demonstrate that, although it is a rare occurrence, aneurysms can involute. We discuss potential mechanisms of this phenomenon and review the literature on aneurysm regression.
Angiographic regression of an aneurysm is a rare occurrence. Most of the reports in the literature have described aneurysms that have ruptured, and a significant portion of them have also involved vasospasm. In this case report, we present a unique case of an unruptured small paraclinoid internal carotid artery (ICA) aneurysm that disappeared after preceding endovascular treatment of a larger concurrent ICA aneurysm. A mechanism for this extraordinary event is postulated, and the literature on regressing aneurysms is reviewed.
Case Report
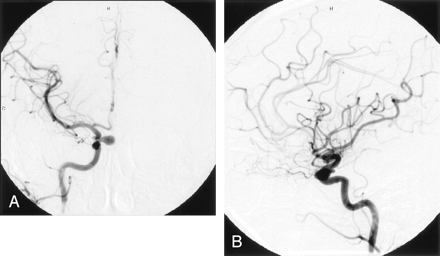
A 43-year-old right-handed white woman underwent MR imaging of the brain because of a cerebellar hemorrhage. There was no evidence of subarachnoid (SAH) or intraventricular hemorrhage on MR images or noncontrast CT scans of the head. The hemorrhage was secondary to overdrainage of CSF via a lumbar subarachnoid drain placed because of inadvertent durotomy during lumbar decompression and fusion surgery. A right ICA aneurysm was suspected on the basis of MR imaging findings. Following full recovery from her parenchymal hematoma, she underwent diagnostic cerebral angiography that demonstrated two cerebral aneurysms in the paraclinoid region of the right ICA. The first aneurysm, a superior hypophyseal artery aneurysm, measured 9 mm and projected medially from the ophthalmic segment. The second aneurysm, originating on the ventral ICA wall proximal to the anterior choroidal artery, measured 4 mm and extended laterally and inferiorly (Fig 1A and B).
AP (A) and lateral (B) right ICA angiograms show a 9-mm superior hypophyseal aneurysm and a 4-mm ventral paraclinoid ICA aneurysm before coil embolization.
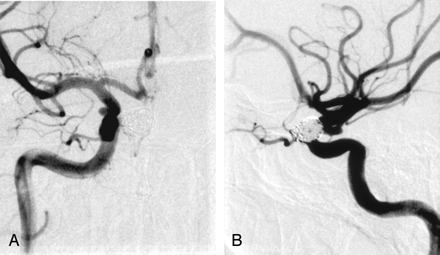
The patient underwent coil embolization with the goal of treating both aneurysms at one time. A combination of Guglielmi detachable coils (Boston Scientific, Fremont, CA) and hydrocoils (Microvention, Aliso Viejo, CA) was used to embolize the more proximal and larger aneurysm without complication (Fig 2A and B). Although the microwire and microcatheter were maneuvered inside the second aneurysm, a stable microcatheter position in the second aneurysm was never attained. After a significant number of attempts, coil embolization of the second aneurysm was postponed. Full heparinization, which was begun after placement of the access sheath in the right common femoral artery, was continued overnight. The patient was discharged on aspirin from the hospital on the first postprocedure day.
AP (A) and lateral (B) right ICA angiograms obtained immediately after coil embolization of the larger superior hypophyseal aneurysm. The smaller, ventral paraclinoid ICA aneurysm remains unchanged.
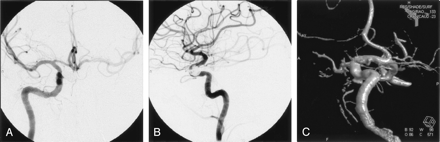
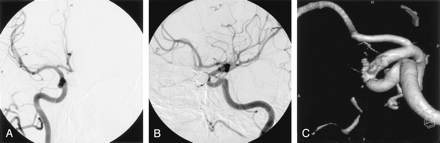
Treatment of the second aneurysm by using a balloon- or stent-assisted technique was planned for 10 weeks later. Aspirin and clopidogrel were prescribed for the 5 days prior to the patient’s second treatment day. Baseline angiograms, including a 3D rotational angiogram, demonstrated complete involution of the aneurysm (Fig 3A–C). The patient returned for a follow-up angiogram at 10 months, which confirmed permanent regression of the aneurysm (Fig 4A–C).
AP (A), lateral (B), and 3D rotational (C) right ICA angiograms at 10 weeks display complete regression of the previously visualized ventral paraclinoid ICA aneurysm.
AP (A), lateral (B), and 3D rotational (C) right ICA angiograms at 10 months display a stable coil configuration within the superior hypophyseal artery aneurysm and continued absence of the ventral paraclinoid ICA aneurysm.
Discussion
It is well known that mycotic aneurysms, arteriovenous malformation–associated aneurysms, and post-traumatic pseudoaneurysms can regress spontaneously (1–5). The incidence of spontaneous thrombosis of saccular aneurysms is unknown, however, and estimates from various autopsy series have ranged anywhere from 0.01% to 13% (6–8). A serial angiographic review at the Karolinska Institute between 1964 and 1973 revealed only one (1.3%) in 78 patients with a spontaneously disappearing aneurysm (9). These authors believe that the true incidence is probably 1–2%. Because the philosophy for the timing of aneurysm surgery after SAH has changed over the past 20 years, any contemporary series would probably underestimate the true incidence of spontaneous thrombosis.
The first report of a spontaneously disappearing aneurysm has been attributed to Marguth and Schieffer (10) in 1957. Our review of the literature revealed a total of 23 cases of angiographically proven saccular aneurysms that appeared to spontaneously disappear on the basis of follow-up angiography findings (Table 1; 9–28). This does not include reports of aneurysms that reappeared at subsequent angiography, presented with thrombosis (i.e., no angiogram before thrombosis), were found to be thrombosed at surgical exploration (i.e., without a follow-up angiogram), or were partially thrombosed (22, 29–31). To the best of our knowledge, there are no case reports that describe an aneurysm that regressed after treatment of a concurrent aneurysm. Furthermore, our case is unique in that the concurrent aneurysm was treated by endovascular means.
Aneurysmal regression series
On closer examination of the literature, there appear to be two separate categories of spontaneously regressing saccular aneurysms—a group that disappeared after SAH and a group that thrombosed without an antecedent rupture. In the SAH group, the two factors that appear to play a role in subsequent thrombosis are the development of vasospasm (with or without parent vessel thrombosis) and treatment with epsilon amino-caproic acid. It is not difficult to believe that significant vasospasm could cause alteration in the hemodynamic forces within the aneurysm to the point at which stasis and thrombosis occur. In fact, some cases have reported thrombosis of the parent vessel along with the aneurysm (13, 17, 19, 22). Similarly, epsilon amino-caproic acid possesses an antifibrinolytic mechanism of action that may predispose the aneurysm to spontaneous thrombosis. Another factor that has been postulated as a contributing factor but has not been well documented in the preceding case reports is systemic hypotension. In fact, there are some early reports of using controlled hypotension to induce spontaneous thrombosis of saccular aneurysms (32, 33).
Although more rare in occurrence, a group of patients exists who had spontaneous regression without antecedent SAH. Within this group, few reports of large or giant aneurysms disappearing after extracranial-intracranial bypass without accompanying parent vessel occlusion can be found (26, 27). In these cases, the plan was to treat the patient with proximal vessel balloon occlusion subsequent to the external carotid artery-middle cerebral artery bypass. Follow-up angiograms, however, showed spontaneous thrombosis of the aneurysms with thrombosis of the parent vessel as well. It was postulated that the high-flow saphenous vein bypass resulted in retrograde filling of the ICA and coupled with the unaltered antegrade flow along the ICA resulted in significant turbulence at the aneurysm site, leading to subsequent thrombosis. The same result, spontaneous aneurysm thrombosis, is usually obtained if the proximal vessel is occluded at the same time the bypass is being performed.
Aneurysm characteristics such as size, neck width, and location have all been considered as potential predictors of spontaneous regression. Stehbens (34) believed that the size of the aneurysmal sac in relation to the caliber of the parent vessel was important in determining the risk for aneurysmal thrombosis. In an experimental model, Black and German (35) demonstrated that the ratio of the aneurysmal volume to the neck width was important with a large volume and a narrow neck predisposing the aneurysm to thrombosis. Most of the regressing aneurysms listed in the Table were medium to large with narrow necks.
On examination of the list of aneurysm locations, there appeared to be few aneurysms found at either the ICA terminus or basilar terminus. It is well established from the endovascular literature that these are the sites where there is significantly increased risk for coil compaction secondary to the pattern of flow into the aneurysm (36). Therefore, it would be logically less likely for an aneurysm in one of these locations to thrombose spontaneously.
After considering all of the above factors, it is apparent that our disappearing aneurysm is different in many respects. Our aneurysm was a small, relatively broad-necked sidewall aneurysm, not a medium to large, narrow-necked aneurysm like most the cases described in the literature. Nor had our patient had an SAH. We postulate, however, that our previous embolization procedure may have somehow altered the hemodynamic flow into the smaller aneurysm, leading to stasis within the aneurysm and subsequent thrombosis. Perhaps the outflow zone of the proximal aneurysm led to the initial formation of the smaller distal aneurysm and, with the obliteration of the proximal aneurysm with coils, the hemodynamic flow was altered significantly enough to result in regression of the distal aneurysm either by changing of the vessel contour or thrombosis. In addition, it was possible that microcatheter or guidewire manipulation within the smaller distal aneurysm may have caused intimal injury promoting thrombosis of the aneurysm. This was less likely, however, because the patient underwent full anticoagulation at the time of the procedure.
Another pertinent issue with regressing aneurysms is the length of follow-up necessary to confirm that the aneurysm does not reappear. In fact, there have been three reports of aneurysms that have angiographically disappeared and subsequently reappeared at follow-up angiography (18, 29, 37). All three cases occurred in the setting of SAH, and in two of the three cases (18, 37) vasospasm appeared to contribute to the nonvisualization, whereas in the remaining case systemic hypotension at the time of the negative angiogram may have played a role (29). In all three cases, the aneurysm reappeared between 1 and 2 weeks after the complicating factors had receded.
Benedetti et al (23) described a similar case in which a ruptured anterior communicating artery aneurysm was demonstrated on an initial angiogram and subsequently re-bled. At follow-up angiography after the re-bleed, no aneurysm opacification was seen despite adequate technical conditions (i.e., no spasm or shifting of vessels secondary to mass effect). On surgical exploration, however, a patent aneurysm was discovered and a clip placed. Another case reported by Atkinson et al (31) involved a fusiform posterior cerebral artery aneurysm that resulted in neurologic deficits related to acute thrombosis of the aneurysm. There was no prethrombosis angiogram documenting the patency of the aneurysm, although a follow-up angiogram obtained 3 months later demonstrated recanalization of the lesion.
Unfortunately, none of these cases can provide any guidance in that they do not match the circumstances of our particular patient, because our patient’s aneurysm was unruptured and was saccular in nature. The 10-month follow-up angiogram obtained in our patient did not show recurrence of the ventral paraclinoid aneurysm. Because our patient had another concurrent aneurysm in which a coil was placed and we characteristically repeat angiography for 2 years after coil placement to monitor for coil compaction and regrowth, we plan to obtain at least one more angiogram. It remains to be seen whether the smaller aneurysm will reappear or whether the cure is durable.
Conclusion
We report on a unique case of an ICA aneurysm that disappeared after endovascular treatment of a concurrent ICA aneurysm. The hemodynamic alterations caused by the embolization procedure may have contributed to the regression.
References
- Received May 1, 2004.
- Accepted after revision July 16, 2004.
- American Society of Neuroradiology