Abstract
BACKGROUND AND PURPOSE: Concern exists that vertebral bodies augmented with cement placed laterally may be at risk of collapse on the nonaugmented side. The purpose of the current study was to determine if placing the cement laterally rather than centrally resulted in risk of collapse on the nonaugmented side.
METHODS: Vertebral bodies (L2–L5) were harvested from eight osteoporotic female cadaver spines. Simulated vertebral-body compression fractures were created and stabilized by injecting 3.5 or 7.0 mL of cement centrally or laterally. Vertebral bodies were recrushed to measure their augmented strength and stiffness. Anterior, posterior, left, and right lateral heights were measured initially, after augmentation, and after recompression.
RESULTS: Lateral and central 3.5-mL injections of cement restored strength, whereas 7.0-mL injections significantly increased strength compared with initial values. The stiffness of vertebral bodies receiving central 3.5-mL injections was significantly less than it was initially, although the stiffness of bodies receiving 3.5-mL laterally was not significantly different from initial values. Initial and posttreatment stiffness values did not significantly differ in vertebral bodies receiving 7.0-mL, lateral or central injections. Vertebral heights did not significantly differ between the augmented and the final compression states in any location. Height loss between central and lateral injections did not differ significantly.
CONCLUSION: Vertebral bodies in which cement is placed laterally do not appear to be at risk for collapse on the unaugmented side.
As percutaneous vertebroplasty becomes more widely practiced to stabilize vertebral body compression fractures, refinements of the procedure are being proposed. One such refinement concerns optimizing the amount of cement needed for stabilization. An early report on vertebroplasty indicated that as much as 15 mL of cement was injected into a single vertebral body (1). Recent biomechanical data suggest that cement volumes on the order of 30% of the vertebral body volume are sufficient (2). Depending on the vertebral level, 30% ranges between 4 and 8 mL. Clinically, volumes as small as 1 mL have reportedly resulted in pain relief and satisfactory outcomes (3). Another refinement of the procedure concerns injecting cement unipedicularly. Using only one site through which cement is introduced theoretically reduces the risk of infection and procedure time relative to bipedicular injection. However, unipedicular injection may result in lateral placement of cement, which may pose the risk of vertebral collapse on the nonaugmented portion of the vertebral body (4). Ex vivo data suggest that such a preferential collapse is not likely (5); however, that study was conducted by using cement volumes greater than those currently used in clinical practice (3).
Therefore, the purpose of the current study was to determine what effect cement volume and cement placement has on mechanical augmentation of compressed vertebral bodies and to determine the risk of collapse on the nonaugmented side of vertebral bodies augmented with laterally placed cement.
Methods
Eight lumbar (L2–L5) spines were harvested from elderly female cadavers (mean age ± SD, 80 ± 9 years). All spines were osteoporotic (mean T score ± SD, −4.0 ± 1.0), as determined by means of dual-energy X-ray absorptiometry (2). The vertebrae were disarticulated, their disks were excised, and the posterior elements were removed (5). The vertebral bodies were wrapped in saline-soaked gauze, sealed in plastic bags, and stored at −20°C until the day before testing. All specimens were thawed at room temperature for 24 hours before testing.
An impression of the endplates of each vertebra was made to distribute the axial load during compression tests (5). Initial interior, posterior, and left and right lateral vertebral body heights were measured by using digital calipers accurate to 0.01 mm (Mitutoyo Corp., Aurora, IL). In the first spine, each vertebral body was arbitrarily assigned to one of the four treatment groups: 3.5 mL central, 3.5 mL lateral, 7 mL central, or 7 mL lateral. The assignment was permutated for each subsequent spine tested. In this manner, the vertebral bodies from each level were evenly distributed among the treatment groups.
Each vertebral body was seated between its respective impressions, which were placed between platens on a materials testing machine (MTS 858, Eden Prairie, MN) and compressed at a rate of 5 mm/min, as described elsewhere (6), to create simulated compression fractures (5). The hydraulic actuator compressed the vertebral body until a decrease in load magnitude was noted, at which time the test was suspended. Such a decrease in compressive load accompanied by increasing displacement was defined as the failure load. Load and displacement data were recorded at 10 Hz.
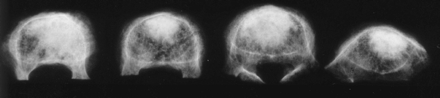
Once the simulated compression fractures were created, 11-gauge cannulae were inserted transpedicularly. For bipedicular cement injection, the cannulae were inserted to converge near the midline in the anterior third of the vertebral body. Vertebral bodies in this group were considered to have received a central injection. The injected volume was either 3.5 or 7.0 mL of polymethylmethacrylate cement (Simplex P, Howmedica Osteonics, Mahwah, NJ). For unipedicular cement injection, the cannula was inserted through the right pedicle into the anterior third of the vertebral body but lateral to the midline (Fig 1). The injected volume was 3.5 or 7.0 mL. Vertebral bodies in this group were considered to have had lateral injections and were chosen to simulate unintentional cannula placement more lateral than clinically desired.
Osteoporotic vertebral bodies were injected (from left to right) with 3.5 mL laterally, 7.0 mL laterally, 3.5 mL centrally, or 7.0 mL centrally.
After the appropriate volume of cement was injected, the vertebral bodies were wrapped in gauze soaked in saline and stored at 4°C for 24 hours to allow complete polymerization of the polymethylmethacrylate cement before retesting. Height (cemented) was measured as before for each vertebral body. Each vertebral body was recompressed according to the initial crush protocol. After the final compression test was done, postcompression height was measured as before for each vertebral body. Stiffness (slope of the load-vs.-deformation curve between 444 N and one-half failure load) and strength were determined for each vertebral body. Strength was defined as the load after which the load trace decreased with increasing compression (5).
We checked for an effect of treatment and time (initial, final) on vertebral-body stiffness, strength, and height measurements (anterior, posterior, and left lateral and right lateral) by using a repeated-measures analysis of variance. The treatment factor had four levels: 3.5 mL central, 3.5 mL lateral, 7.0 mL central, and 7.0 mL lateral. For strength and stiffness, the time factor had two levels: initial and final. For height measurements, the time factor had three levels: initial, augmented, and final compression. Differences between groups were checked for significance by using Tukey’s post-hoc test. Significance was defined as P < .05 (unless otherwise specified).
Results
We observed no significant differences in the initial strength values of the vertebral bodies in the four treatment groups (Table). Strength was restored to vertebral bodies injected with 3.5 mL of cement, regardless of the injection site (lateral or central). Strength was significantly increased in vertebral bodies injected with 7.0 mL of cement, regardless of injection location (lateral or central). Stiffness of vertebral bodies centrally injected with 3.5 mL was significantly less than it was initially. Although the stiffness of vertebral bodies laterally injected with 3.5 mL was also less, the difference was not significant. Initial and posttreatment stiffness values did not significantly differ in vertebral bodies injected with 7.0 mL, regardless of injection site (lateral or central).
Restoration of mechanical properties with vertebroplasty
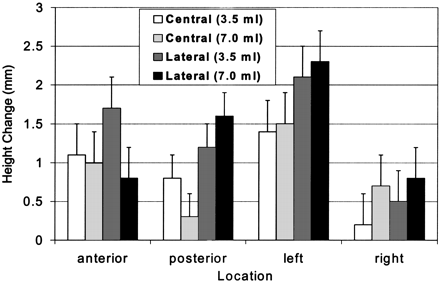
Heights of the vertebral body did not significantly differ between the augmented state and the final compression state at any location (anterior, posterior, left, and right) (Fig 2). In general, height loss was greater on the left side, but the loss was not significantly different from that on the right side. Furthermore, height loss on the left did not differ between specimens injected in both pedicles and those injected in the right pedicle; therefore, height loss did not appear to be treatment related.
Mean change in height measurements immediately after cement augmentation and immediately after final compression. None of the changes was significantly different.
Discussion
The main motivation of the current study was to determine if vertebral bodies treated with unipedicular injections preferentially collapse on the noninjected side. Such a phenomenon would create a lateral wedge and might result in a deleterious alteration of the normal kinematics of the spine. Our results indicated no significant difference in left vertebral body height between the treatment groups. In fact, we observed no significant difference in height changes between any of the treatment groups in any of the four locations measured. Therefore, our original concern that unipedicular-injected vertebral bodies would preferentially deform on the uninjected side was unfounded. Height loss was greater on the left than on the right, but this loss was roughly the same in all treatment groups, and differences were not statistically significant. Type II error was possible because the power of these comparisons was low (β ≈ 0.1). Even if differences in left- and right-sided height loss were significant, they do not appear to be related to treatment (either the site of injection or the volume), and they were on the order of 1–2 mm. It is doubtful that such a small loss of height is clinically important. Therefore, preferential height loss on the nonaugmented side appears to be more of a theoretical concern (4) than a practical one.
Treatment with either lateral or central injections of 3.5 mL restored vertebral-body strength, whereas injections of 7 mL increased vertebral-body strength relative to initial values. Only stiffness of vertebral bodies injected centrally with 3.5 mL exhibited a significant decrease in stiffness relative to initial values. An argument can be made that restoration of strength is a clinically desirable goal of vertebroplasty. Because the surrounding unaugmented cancellous bone is still osteoporotic, attaining an augmentation stronger than the surrounding bone results in fracture of the surrounding bone. The stiffness of the augmented vertebral body is likely a more important parameter. Sufficient stiffness provides stability to the fractured vertebral body, prevents micromotion, and is likely responsible for the pain relief after vertebroplasty. In long bones, restoration of 20–80% of the original stiffness results in a mechanical environment conducive to fracture healing (7). The reparative benefit of cement augmentation appears to be related more to the volume injected than to where it is placed. This finding is consistent with previous reports (5) suggesting no significant collapse on the noninjected side. It is also consistent with previous publications suggesting that, in the lumbar spine, as little as 2 mL can restore strength (8), although a subsequent report suggested that 6 mL is needed (2).
Stiffness was restored by injecting 7 mL of cement in both the lateral and the central groups. This finding is consistent with that of a previous study (8), but a second related study suggested that stiffness is not restored in the lumbar region, even with 8 mL of cement (2). In the current study, the 3.5-mL lateral injection restored stiffness unexpectedly, whereas the central 3.5-mL injection did not. Although initial stiffness values were not significantly different between groups, initial stiffness for vertebral bodies receiving 3.5-mL central injections was approximately 30% greater than that of those assigned to the 3.5-mL lateral injection group; this factor likely influenced the repair. In fact, our results and those of past studies indicate substantial variation in mechanical behavior between vertebral bodies. There is also a weakly correlated dose-response relationship between the volume of cement injected and the stabilization it provides (2). Because of this weak correlation, it may be difficult to precisely predict the amount of cement needed to stabilize these fractures clinically. We used Simplex P, but other polymethylmethacrylate and hydroxyapatite cements are being used for vertebroplasty in clinical settings(9–13). Differences in cement properties likely play a smaller role in vertebral body augmentation than cement volume and placement (14).
Our study was limited by the constraints of any investigation in cadaveric tissue. We assume that mechanical stabilization results in the pain relief provided by vertebroplasty and that most patients would tolerate a height difference of 1–2 mm from the right lateral aspect of the vertebral body to the left lateral aspect without untoward effects. Clearly, these assumptions cannot be tested in a cadaveric model. Clinically, small volumes (about 2 mL) of cement have been injected and have resulted in pain relief (3, 15–17). Cement has also been injected via a unipedicular route clinically (16). To our knowledge, no carefully controlled prospective randomized study has been conducted to show a dose-response relationship for cement used in vertebroplasty, nor has any controlled study been done to compare the efficacy of unipedicular vertebroplasty with that of bipedicular vertebroplasty. Although no prospective randomized controlled study has established the efficacy of vertebroplasty (18), results from one nonrandomized study suggest that vertebroplasty results in early pain relief; however, at 6–12 months, treated and nontreated patients have similar clinical outcomes (3).
Conclusion
The current results suggest that unipedicular injections of as little as 3.5 mL of cement placed laterally in an osteoporotic vertebral body do not lead to preferential collapse on the unaugmented side. The report is not intended to promote lateral placement of small volumes of cement, but rather, to demonstrate how robust vertebroplasty is in stabilizing the spine, even when small volumes of cement are inadvertently placed in clinically less-than-desirable areas.
Acknowledgments
The authors thank Howmedica Osteonics for donating the cement used in this study and Charlene Wu for her assistance with specimen preparation.
References
- Received February 26, 2004.
- Accepted after revision May 12, 2004.
- Copyright © American Society of Neuroradiology