Abstract
BACKGROUND AND PURPOSE: The abducent nerve is difficult to identify reliably and consistently with conventional radiologic techniques. In this study, a 3D fast asymmetrical spin-echo MR imaging technique was used to obtain detailed images of the abducent nerve in normal volunteers.
METHODS: The 3D fast asymmetrical spin-echo MR protocol was used to image the abducent nerves in 24 normal volunteers by using a 1-mm section thickness in the tilted axial and parasagittal planes. The microanatomy of the abducent nerve within Dorello’s canal was also demonstrated in a cadaver study.
RESULTS: In 24 normal volunteers, the anatomy of 47 abducent nerves was clearly depicted on MR images. The length of the cisternal segment of the abducent nerve, extending from the brain stem to its dural foramina, ranged from 6.7 to 19.6 mm (mean, 13.1 mm). The abducent nerves were at an angle of 5 to 90 degrees (mean, 24.5 degrees) to the clivus. CSF evagination was detected in the region of Dorello’s canal in 36 (77%) of 47 abducent nerves. The length of CSF evagination varied: 0.9 mm in five nerves, 1.0 to 1.9 mm in 18 nerves, 2.0 to 2.9 mm in eight nerves, and 3.0 mm or more in five nerves. Histologic examination of serial sections of the abducent nerve revealed that the petroclival segment of the nerve was covered by an envelope composed of an arachnoid cell layer.
CONCLUSION: The course of the abducent nerve was reliably identified using the 3D fast asymmetrical spin-echo MR protocol and a histologically proven arachnoid envelope around the petroclival segment of the nerve was shown as CSF evagination into Dorello’s canal by MR imaging.
The abducent nerve, cranial nerve VI, innervates the lateral rectus muscle of the eye and is responsible for lateral horizontal ocular movement. Although the abducent nerve passes a long distance from the brain stem to the lateral rectus muscle, it is difficult to identify this nerve reliably and consistently by using conventional radiologic techniques. The recent advent of MR imaging techniques, however, has improved our ability to examine various cranial nerves (1, 2) and has made it possible to clearly visualize the abducent nerve. This report describes detailed images of the normal anatomy of the abducent nerve, obtained by using a 3D fast asymmetrical spin-echo MR imaging technique, with special emphasis on CSF evagination into Dorello canal.
Methods
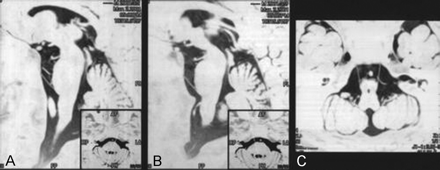
Twenty-four normal volunteers (15 men and nine women; age range, 19−63 years) were studied by using the 3D fast asymmetrical spin-echo MR imaging protocol on a 1.5-T MR imaging system (VISART; Toshiba Corporation, Tokyo, Japan). The imaging parameters used were 6000/80 (TR/TE); section thickness, 1 mm; matrix, 224 × 256; and number of acquisitions, 1. Axial view images were first examined to identify the cisternal segment of the abducent nerve, and parasagittal and tilted axial view images obtained parallel to its course were then obtained (Fig 1). The time required for a single shot was approximately 5 min 30 s.
MR images of a 42-year-old man were obtained in the parasagittal (A and B) and titled axial (C) planes using a 3D fast asymmetrical spin-echo sequence. Images are displayed in reverse. Axial view images were first examined to identify the cisternal segment of the abducent nerve. Parasagittal (A and B) and tilted axial (C) view images were then obtained parallel to its course. The entire course of the cisternal segment of the abducent nerve could be identified. The nerves on both sides were visualized in the same tilted axial plane.
The abducent nerve and surrounding dura mater of the petroclival region were removed from two adult cadavers prepared for a dissection course at Jutendo University after vascular injection of formalin. After embedding in paraffin, serial sections of the specimens obtained perpendicular to the nerve were stained with hematoxylin and eosin.
Results
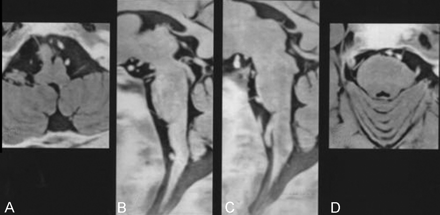
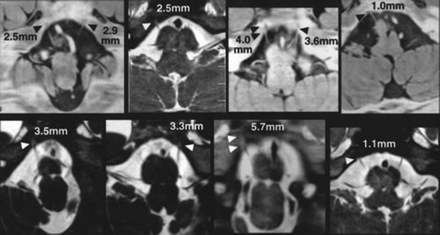
In 24 normal volunteers, the anatomy of 47 abducent nerves was clearly depicted in the axial plane as well as in the parasagittal and tilted axial planes (Figs 1–3). The nerve ran in contact with the ventral surface of the pons in most cases, and the origin of the nerve at the brain stem could not be clearly identified. The length of the cisternal segment of the abducent nerve, which ranged from 6.7 to 19.6 mm (mean, 13.1 mm), was measured as the nerve emerged from the pontomedullary sulcus. The abducent nerves were at an angle of 5° to 90° (mean, 24.5°) to the clivus. CSF evagination was detected in the region of Dorello canal in 36 (77%) of 47 abducent nerves (Fig 3). The length of CSF evagination varied: 0.9 mm in five nerves, 1.0 to 1.9 mm in 18 nerves, 2.0 to 2.9 mm in eight nerves, and 3.0 mm or more in five nerves.
MR images of the abducent nerves in a 27-year-old man were obtained by using the 3D fast asymmetrical spin-echo sequence. Black and white reversed images are shown. A and D, tilted axial view images; B and C, parasagittal view images. The right abducent nerve was 30° to the clivus (A and B), and the left abducent nerve was 90° to the clivus (C and D).
CSF evagination into Dorello canal on tilted axial view images of eight volunteers. Dorello canal can be identified as a CSF-filled evagination of variable length (arrowheads). The CSF evagination ranged from 1.0 to 5.7 mm in these eight volunteers.
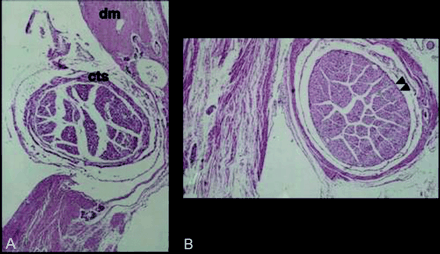
Histologic examination of serial sections of the abducent nerve revealed that the petroclival segment of the nerve was surrounded by a fluid-filled space, which was considered an extension of the subarachnoid space (Fig 4). This extension of the subarachnoid space continued along the entire course of the petroclival segment of the abducent nerve. In the initial part, at the porus duralis, the abducent nerve together with moderately attenuated connective tissue was invested in the envelope of arachnoidal loose connective tissue (Fig 4A). In the middle part of the Dorello canal, the nerve covered by a compact sheath was invested in a connective tissue envelope composed of two layers: an inner loose layer of arachnoidal extension and an outer attenuated layer of dural extension (Fig 4B). This inner arachnoidal connective tissue contained a substantial fluid-filled space, which obviously was a continuation of the subarachnoid space at the porus duralis.
Light micrographs of cross sections of the abducent nerve.
A, Cross section obtained at the porus duralis. The abducent nerve at the opening of attenuated connective tissue of the dura mater (dm) was covered by a moderately attenuated connective tissue sheath (cts). The loose connective tissue and fluid-filled space outside the connective tissue sheath represents the arachnoidea and subarachnoid space, respectively.
B, Cross section obtained at the midportion of the petroclival segment. The abducent nerve within the Dorello canal was covered by a compact sheath (arrowheads). It was invested in two layers of connective tissue envelope, consisting of an inner loose arachnoidal and an outer attenuated dural extension.
Discussion
The abducent nerve arises from the abducent nucleus and exits the brain stem at the pontomedullary sulcus. The nerve then runs forward and laterally within the subarachnoid space to its opening in the dura (cisternal segment of the abducent nerve). Marinkovic et al (3) examined 14 cadaver brains to observe the anatomic characteristics of the cisternal segment of the abducent nerve. They found that the initial part of the abducent nerve was most often located within the pontomedullary sulcus and that several rootlets promptly left the sulcus to form a more or less compact trunk of the abducent nerve. In most of their cases, all the rootlets exited from the pontomedullary sulcus. In 17.86% of the nerves, however, from one to three rootlets were seen to emerge from the caudal part of the pons, immediately rostral to the pontomedullary sulcus. Only one (3.57%) of the nerves was observed to exit completely from the caudal pons. We found that the abducent nerve ran in contact with the caudal pons at the beginning of its course in cases in which the nerve was at an acute angle to the clivus. In such cases, the nerve appeared to emerge from an area rostral to the pontomedullary sulcus, but it is impossible to ascertain the validity of this finding based on 3D fast asymmetrical spin-echo MR images. In the present study, only one nerve could not be captured on the first axial section. Although the exact cause of this not known, it could be attributable to the physical proximity of the nerve to a blood vessel, artifacts caused by pulsation of the CSF, or an unexpected innervation pattern.
After the abducent nerve reaches the dural opening, the nerve passes the basilar plexus, a space located between the two dural leaves (petroclival segment). Sheaths of dura and arachnoid accompany the nerve through its portal into the basilar plexus. After its passage through the basilar plexus and subsequently through the inferior petrosal sinus, the nerve runs below Gruber’s ligament (the petrosphenoidal ligament) into the upper posterior region of the cavernous sinus. Enveloped in its dural, arachnoid, and connective tissue sheaths, it then runs rostrally, lateral to the internal carotid artery (4). Classic works have defined Dorello canal as the small space located between the petrous apex and Gruber’s ligament (5, 6). Other authors, however, have described it as a larger space located between the two dural leaves and extending from the point where the abducent nerve pierces the dura mater to its entrance into the cavernous sinus (7, 8). We agree with the latter description and think that Dorello canal could also be viewed as a CSF-filled invagination into the petroclival dura matter. Destrieux et al (7), who also agreed with the latter description, stated that Dorello canal was a larger space that should be named the petroclival venous confluence. In addition, they found that the abducent nerve was surrounded by a sheath composed of arachnoid tissue or dura mater and that this meningeal sheath followed the nerve to the cavernous sinus. Yousry et al (9) found that CSF evagination was evident in the region of Dorello canal in 94% of abducent nerves examined by MR imaging cisternography. We also observed CSF surrounding the petroclival segment of the abducent nerve in 77% of the nerves examined by using a 3D fast asymmetrical spin-echo imaging technique. This finding is supported by the existence of an arachnoid cell layer surrounding the nerve that was evident in cadaver specimens. Using our 3D fast asymmetrical spin-echo imaging protocol, a layer of CSF <0.5 mm in thickness might be overlooked, so the apparent lack of CSF shown on MR images does not necessarily indicate the absence of a subarachnoid space surrounding the nerve. We conclude that the petroclival segment of the abducent nerve is covered by an envelope of arachnoid cells and an extension of the subarachnoid space over its entire course.
With the 3D fast asymmetrical spin-echo imaging method, it was possible to clearly capture the abducent nerve from the cisternal segment to the petroclival segment, thus providing reference for the normal innervation pattern of the abducent nerve. In patients with a compressive or space-occupying lesion in this region, such as petroclival meningioma or chondroma, the anatomic relationship between the abducent nerve and the lesion can be assessed by using this method. Also, in patients with abducent paralysis, information regarding the normal innervation pattern of the abducent nerve is useful for ruling out organic abnormality of the nerve up to Dorello canal.
Conclusion
The course of the abducent nerve can be reliably identified by using the 3D fast asymmetrical spin-echo MR imaging protocol. A histologically proved arachnoid envelope around the petroclival segment of the nerve was shown as CSF evagination into Dorello canal by MR imaging.
Acknowledgments
We thank Kazuko Yokomizo for technical assistance in preparing serial sections of the specimens from cadavers.
- Received April 5, 2003.
- Accepted after revision September 2, 2003.
- Copyright © American Society of Neuroradiology