Abstract
BACKGROUND AND PURPOSE: Head CT prescriptions are currently plagued by intra- and intersubject image variance and do not match standardized MR imaging planes. We developed and tested a simple method to improve CT precision and approximate the Talairach reference standard advocated for MR imaging.
METHODS: We retrospectively reviewed midline sagittal T2-weighted brain MR images of 126 consecutive patients to determine the mean angle subtended by the Talairach anterior commissure-posterior commissure (AC-PC) line and the hard palate. On the basis of this data set, a new head CT protocol was instituted with pitch similarly prescribed relative to the hard palate as identified on the lateral CT scout film. We then compared the precision of the new protocol, our former method (nominally parallel to the orbito-meatal line) and fixed-gantry angulation. Two head CT studies from 50 consecutive patients imaged with our old protocol and 50 consecutive patients imaged with our new protocol were reviewed for a total of 200 CT examinations.
RESULTS: The Talairach AC-PC line was rotated 12.0° ± 6.1° from the hard palate line and 15.6° ± 10.1° from the axial plane of the magnet. The new CT protocol approximated the Talairach-referenced MR images obtained at our institution and improved intrapatient CT scan precision compared with fixed-gantry selection (P < .004) and compared with our previous prescription technique (P < .064; P < .025, controlling for excessive head extension).
CONCLUSION: By prescribing CT images angled +12° from the hard palate, a structure readily identified by technologists, interscan precision can be improved and Talairach-referenced MR imaging studies can be approximated. Along with AC-PC-referenced MR imaging studies, we advocate this CT protocol as a new clinical standard.
Variability in head positioning and prescribed techniques for MR imaging and CT may yield significant intra- and intersubject image variance within and across modalities. Having already become a de facto standard for neuroscience research and stereotaxis, the Talairach reference has been recently advocated as a new standard for clinical brain MR imaging (1–4). Recently, both technologist- and computer-driven methods to directly prescribe Talairach-referenced MR images have demonstrated a substantial reduction in interpatient scan variance and more efficient brain coverage than routine clinical axial imaging (3).
CT head scans have been traditionally prescribed parallel to the orbito-meatal line (OML), defined as passing through the lateral canthus and middle of the external ear canal (5–7). Use of this external reference line for CT prescriptions, however, has several major drawbacks. First, it is difficult to discern the OML on the lateral scout view from which technologists currently prescribe. Second, if a patient’s head is extended, scanner limitations in gantry angulation (eg, 22° for most GE CT scanners [General Electric Corporation, Milwaukee, WI]) may preclude accurate OML prescription. These two factors can lead to significant prescription errors and inter- and intrasubject CT image variance. In addition, the OML may have a relatively inconstant relationship to superficial and deep brain structures, resulting in further intersubject variance in the appearance of the brain on axial CT sections (8). Finally, the OML matches neither conventional MR axial sectioning nor Talairach anterior commissure-posterior commissure (AC-PC)-referenced imaging, being approximately 24° steeper than the former and 9° steeper than the latter (3). As a result, axial head CT scans parallel to the OML may be difficult to directly compare with axial brain MR imaging sections, particularly those conventionally prescribed orthogonal to the bore of the magnet.
We developed and tested a simple method to improve CT precision and approximate the Talairach reference standard advocated for MR imaging by using the hard palate as a landmark. The hard palate was selected because it is a relatively planar midline structure fixed to the skull and projects as a line on the lateral CT scout film. In addition, this readily identifiable landmark is already used by technologists to prescribe maxillofacial CTs.
Methods
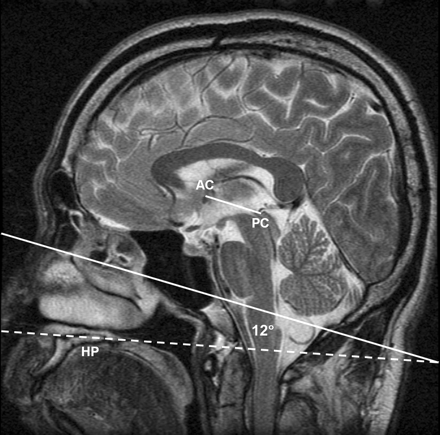
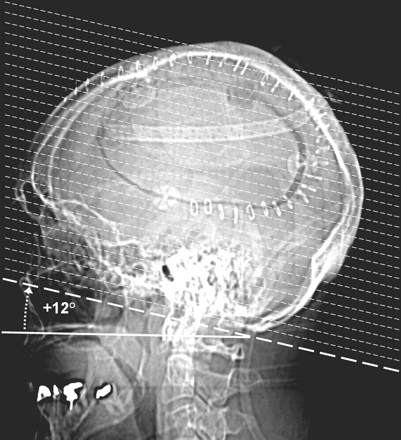
Institutional review board approval was obtained. Subsequently, to determine the mean angle subtended by the Talairach AC-PC line and the hard palate, two coauthors (K.L.W. and J.S.) retrospectively reviewed a data base of 126 midsagittal, T2-weighted clinical brain MR images produced from May 7, 2002 to May 21, 2002 at our institution. (Fig 1) The MR imaging population consisted of 64 male and 62 female subjects ranging in age from 17 to 89 years, with an average age (±SD) of 49.2 (±17.8) (3). On November 15, 2002, we instituted a new clinical head CT protocol (HP+12) in which scan pitch (gantry tilt) was to be offset by this predetermined angle relative to the hard palate as identified by technologists on the lateral CT scout film (Fig 2). This protocol replaced our former protocol (OML*) in which the technologists were instructed to angle the gantry parallel to the OML as visualized on the lateral scout, albeit difficult to identify. The asterisk designates the technologist approximation of the OML from the lateral scout rather than the true anatomic OML.
Midline roll- and yaw-corrected sagittal fast spin-echo T2-weighted MR image (TR/TE, 3816/105eff;echo train length, 16; section thickness, 4 mm; matrix 512 × 256; field of view, 20 cm). The short solid line corresponds to the Talairach AC-PC basal reference; The long solid line is drawn parallel to the Talairach AC-PC reference, and the dashed line passes through the superior cortical surface of the hard palate. Note in this prototypical case the angle subtended by the Talairach AC-PC line and the hard palate is 12°. AC, anterior commissure; PC, posterior commissure; HP, hard palate.
Lateral CT scout view from a study patient illustrating the axial scan prescription (dotted lines) with the HP+12 protocol angled +12° from a line passing through the hard palate (solid line). The solid line indicating the orientation of the hard palate has been offset a few millimeters inferiorly to provide a clear view of the hard palate.
To compare the performance of our new protocol (HP+12) against our former protocol (OML*) and an alternate hypothetical fixed-gantry protocol (AX+15) optimized to approximate the Talairach AC-PC line, we reviewed a total of 200 head CT examinations. These included 50 consecutive patients with two head CT studies taken by using the OML* protocol and 50 consecutive patients with two head CT studies taken with the HP+12 protocol. The CT scan population consisted of 56 male and 44 female subjects. Patient age was only available for 57 of the patients and ranged from 16 to 93 years, with a mean of 49.8 years (±17.6).
For each CT study, the lateral CT scout and technologist selected gantry angle for axial sections were collected for review, the latter obtained from the DICOM header. Hard palate angles relative to the horizontal plane were measured independently on all 200 CT scouts by three coauthors (K.L.W., J.L.W., and W.S.) and were subsequently reviewed together by the same three coauthors en banc to establish a hard-palate angle consensus and determine a gantry detector tilt coauthor-derived criterion standard. For the alternate fixed-gantry protocol, the average hard palate angle was used. The sign convention for angle measurements defines head extension as positive.
Pearson correlation coefficients were compared for the OML* and HP+12 protocols. Precision and accuracy relative to the coauthor criterion standard for each gantry tilt method was also evaluated. The accuracy of a particular study was measured as the absolute difference between the technologist-selected gantry angle and the coauthor criterion standard: 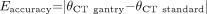 1
1
The precision or reproducibility of each method was calculated as the absolute difference in prescription accuracy for each pair of CT scouts obtained from the same subject:  2
2
To adjust for population bias and isolate the influence of our CT scanner’s 22° maximum gantry tilt limitation, full and reduced data sets for the OML* and HP+12 technologist method protocols were compared. The reduced data set consisted of only those scans requiring a gantry tilt less than or equal to 22° as determined by the coauthor-derived hard-palate criterion standard.
Statistical analysis was performed by using DSTPLAN, NCSS 2001, and Microsoft Excel (9–11). A statistical significance threshold of α = 0.05 was applied for all inferences.
Results
Paired measurements of the Talairach AC-PC reference and hard palate could be obtained for only 117 of the 126 MR imaging studies because of distorted anatomy, artifact, or limited field of view. For these 117 studies, the Talairach AC-PC line was extended by 12.0° (±6.1°) from the hard palate line and 15.6° (±10.1°) from the axial plane of the magnet. Consequently, to approximate the Talairach AC-PC line protocol the HP+12 CT protocol prescribes CT gantry detector tilt at +12° extension from the hard palate as identified by the technologists on lateral CT scout film.
Average technician-prescribed gantry tilt by using the OML* protocol was 11.1° (±8.6°) and 13.4° (±7.5°) by using the HP+12 protocol.
In all 200 CT lateral scout images, a coauthor consensus for the hard palate orientation was achieved. Interauthor correlation coefficients for hard palate angle measurements from the CT lateral scout ranged from 0.90 to 0.95, and individual author correlation with the hard palate criterion standard ranged from 0.94 to 0.98. The hard palate in all lateral scout CTs studied was extended an average 3.0° (±11.9°).
Overall, the standard hard palate extension averaged 3.0° (±11.9°). For the alternate hypothetical fixed-gantry protocol (AX+15) we chose a fixed-gantry tilt of 15° to best approximate the Talairach AC-PC line as extended +12° from the overall average hard palate angulation of 3.0° on lateral scout CT to arrive at a hypothetical fixed-gantry tilt of 15°.
The patient heads studied with the HP + 12 protocol were more extended than those studied with the OML* protocol. This introduced a significant population bias, in view of the 22° maximum gantry tilt permitted on our GE scanners. To compensate for this bias, a reduced data set was created by selecting only CT scans that require gantry prescriptions no greater than 22°. The reduced data set excludes 56 (25 OML* scans and 31 HP + 12 scans) of the 200.
The difference in correlation of the OML* (R = 0.78) and HP+12 (R = 0.82) prescriptions with the coauthor criterion standard was not statistically significant when comparing full data sets (P = .18; power = 0.23; Table 1A). The HP+12 method, however, was more strongly correlated with the criterion standard (R = 0.87) than was the OML* method (R = 0.69) for the reduced data set (P < .003; power = 0.87; Table 1B) Correlation analysis does not apply to the AX+15 fixed-gantry protocol because a constant prescription of 15° was used.
Comparative performance of prescription protocols
Due to the use of absolute values, the distributions of accuracy and precision metrics were significantly nongaussian. To derive inferential statistics, data transformations were tested (12). Cubic-root transformation was found to significantly improve the normalcy of all variables and was subsequently applied.
Both the HP+12 and OML* technologist protocols were more accurate than the fixed-gantry AX+15 alternative (P < .0001). There was no statistical difference between the accuracies of the HP+12 and OML* protocols for the full data set (P = .71; power = 0.07; Table 1A) For the reduced data set, however, the accuracy of the HP+12 protocol was superior to that of the OML* protocol (P = .004; power = 0.83; Table 1B).
The HP+12 protocol was more precise than the fixed-gantry alternative AX+15 protocol (P = .004; power = 0.85). The OML* protocol did not significantly improve precision relative to the fixed-gantry AX+15 protocol (P = .18; power = 0.24). There was also evidence of improved precision by using the HP + 12 protocol relative to the OML* protocol, but this did not demonstrate statistical significance at α = 0.05 for the full data set (P = .06; power = 0.46; Table 1A). For the reduced data set, the precision of the HP+12 protocol was superior to that of the OML* protocol (P = .025; power = 0.63; Table 1B).
Discussion
Institution of the aforementioned CT prescription protocol was easy for our technologists and did not require special training. Concurrently, we changed our routine section thickness from 5 mm through the posterior fossa and 10 mm above to contiguous 5-mm-thick sections through the entire brain. This simplifies brain prescriptions, improves interscan image concordance by reducing maximum offset from 5 to 2.5 mm, permits subsequent whole brain multiplanar reconstructions on our multidetector scanners, and better approximates our routine 4-mm MR imaging brain sectioning. In addition, we reduced our field of view from 25 to 22 cm to increase in-plane spatial resolution and better approximate our MR imaging studies typically performed with a 20-cm field of view.
Although readily identifiable on lateral CT scout films, the hard palate may have some limitations as a landmark for pitch determination. The superior surface of the hard palate appears grossly planar, but curvature may exist (13). Coupled with the relatively short anteroposterior dimension of the hard palate, this curvature could yield some inherent interobserver error. Nonetheless, as hypothesized, using the hard palate as a landmark did improve CT scan precision and accuracy relative to fixed-gantry angulation and to the OML* and AX+15 protocols, the latter nominally relying on the OML. Unexpectedly, the technologist-selected OML* was less extended than the HP+12 line designed to approximate the Talairach AC-PC reference. Two factors could be contributory: either our technologists were choosing landmarks offset from the patient’s actual OML or the patient population did not approximate the 305 healthy volunteer Montreal Neurological Institute brain population in this measurement (3). In light of the difficulty of discerning the OML on lateral CT scout films, we believe the former factor likely predominates, hence the asterisk in the OML* designation.
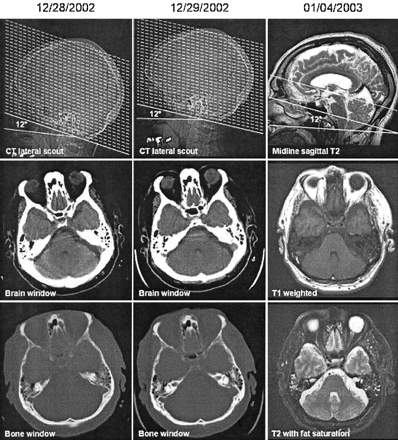
By approximating the Talairach AC-PC line in an individual patient, the HP+12° protocol may be discordant with the Talairach reference, reducing CT-MR imaging intermodality precision. In our study, the angle subtended by the hard palate and the AC-PC line varied from patient to patient (SD = 6.3°). Consequently, excellent CT-MR imaging concordance as demonstrated in Figure 3 would be expected to occur only when an individual’s subtended angle closely approximates the mean of 12°. (Figs 1 and 3)
Consecutive CT and MR examinations from the same subject taken on different days by using the HP+12 CT protocol and a direct Talairach referenced MR imaging protocol (3). First row illustrates axial CT and MR image prescription methodologies. Note in this archetypal patient the angle subtended by the hard palate and the AC-PC line as depicted on the sagittal T2-weighted MR image is 12°, matching the CT prescription protocol. The second and third rows illustrate a representative axial section from these examinations through the orbits and posterior fossa with differing CT windows or MR sequencing. Axial CT and MR sections are 5 mm and 4 mm thick, respectively. Note the excellent intra- and intermodality axial scan concordance.
To improve intermodality image concordance, implementing the HP+12° CT algorithm should ideally be done in conjunction with adoption of the Talairach MR imaging reference standard. If for technical, anatomic, or pathologic reasons technologists can identify the hard palate but not the AC and PC on a patient’s midline sagittal MR imaging, the MR imaging study could be prescribed in a similar fashion to that suggested for brain CT scans; that is, 12° steeper than the hard palate. Theoretically, this should further reduce CT-MR imaging intermodality variance while approximating the desired Talairach AC-PC pitch.
In contradistinction to the three-step protocol proposed by Weiss and coauthors for providing direct Talairach-referenced MR imaging examinations, the CT protocol does not compensate for patient roll and yaw (2, 3). Consequently, to optimize CT results, care should be given to ensure that the patient’s head is not significantly rotated within the head holder/gantry. Moreover, when using a CT scanner constrained by a maximum gantry tilt of less than 30°, significant head extension should be avoided as much as possible. Unfortunately, in the acute trauma setting, technologists may not be able to readily or safely reposition the patient’s head.
In view of the study’s urban trauma level-one medical center setting and the two- CT-examination inclusion criteria, our scan population was strongly biased to acute traumatic injury. Consequently, higher precision might be expected in a different setting, such as an outpatient imaging facility or with CT scanners that permit greater gantry angles. Because our study did not include infants or children, the results may not yet be generalized outside the adult population. Further investigation is currently underway to include evaluation of the proposed methodology in pediatric patients.
Conclusion
By prescribing CT images angled 12° from the hard palate, interscan precision can be improved and Tailarach-referenced MR imaging studies can be approximated. Along with Talairach AC-PC-referenced MR imaging studies, we advocate this CT protocol as a new clinical standard. Adoption of these complementary CT and MR imaging prescription protocols should facilitate intra- and intermodality comparisons, leading to more reproducible and readily interpretable brain imaging findings.
Footnotes
Presented, in part at the American Society of Neuroradiology, April 29, 2003, Washington, DC.
- Received May 28, 2003.
- Accepted after revision August 20, 2003.
- Copyright © American Society of Neuroradiology