“I have a patient that just walked into my clinic. He recently moved into the area and does not have any prior medical records. He underwent a partial glossectomy for an oral tongue carcinoma and a supraomohyoid neck dissection about 18 months ago. He was reconstructed with a myocutaneous flap and underwent postoperative radiation therapy. He now complains of new intermittent tongue pain over the past 3–4 months. I am somewhat concerned about recurrence, but he does not have a palpable mass. What imaging study should I order?”
Sound familiar?
Information from cross-sectional imaging is an important component for initial staging and post-treatment evaluation of the patient with squamous cell carcinoma of the head and beck (HNSCCA). The 6th edition of the American Joint Committee on Cancer staging manual specifically states that “any diagnostic information which contributes to the overall accuracy of the pretreatment assessment should be considered in clinical staging and treatment planning” (1)
There are a variety of imaging modalities that have been shown to be useful for differentiating recurrent tumor from posttreatment changes. These include CT, MR, positron emission tomography (PET), and thallium single-photon emission tomography (SPECT). In addition, there are emerging techniques such as new PET radiotracers and PET-CT. We are now left with the dilemma of imaging recommendations to evaluate the post-treatment neck, and therein lies an inherent controversy within the neuroradiology and clinical community.
The true diagnostic accuracy with which an imaging technique detects recurrent tumor following treatment is difficult to assess. This is based on the clinical suspicion of disease at the time of imaging. Imaging performed in a patient population with a high clinical suspicion of disease based on a palpable mass or suspicious biopsy before imaging will yield a higher diagnostic accuracy as opposed to patients without a palpable mass presenting with equivocal symptoms (2). This important information is often difficult to extract from the literature.
Comparison with old studies is essential for accurate interpretation for all imaging studies. This is especially important for CT. The characteristic CT findings of recurrent tumor are 1) a progressively enlarging mass at the primary site or along the surgical margin or 2) a progressively enlarging lymph node (3, 4). Very advanced tumors may erode bone. The enhancement pattern in recurrent tumors is variable (Fig 1). The task is made even more difficult if old studies are unavailable. A growing number of patients are being treated with multimodality regimens that include a combination of surgery, radiation therapy, and chemotherapy. These post-treatment changes often make the diagnosis of recurrent tumor difficult on the basis of a single study. Accurate identification of recurrent tumor is made even more difficult, because the recurrence often occurs within the distorted anatomic bed of the treated primary site. In general, the literature suggests that CT has a high sensitivity (63–100%) and moderate specificity (24–80%) for differentiating recurrent tumor from post-treatment changes (2, 5–8). The accuracy may be increased in patients treated with nonsurgical organ preservation therapy by comparing the primary site tumor volume on the pre- and post-treatment imaging study. Complete radiologic resolution is indicative of cure, whereas a reduction in size of less than 50% is indicative of treatment failure. Patients with a 50–75% reduction are indeterminate and require close surveillance (9).
The MR imaging criteria for recurrent tumor are an enlarging enhancing infiltrating mass that is of intermediate to high signal intensity on T2-weighted images. Prior studies suggest that abnormal soft tissue that has decreased T2-weighted signal intensity is suggestive of post-treatment scarring rather than recurrent tumor (3, 4, 8, 10, 11). There are numerous published reports on the ability of MR to detect recurrent HNSCCA; however, there is currently a paucity of data commenting on the diagnostic accuracy. The ability of MR to detect recurrence will depend on the experience of the individual interpreting the study (12). There is consensus that MR is the preferred technique (whether anatomic or metabolic) for detecting perineural spread or early intracranial extension as a pretreatment and post-treatment baseline study in patients with skull base tumors. MR should be performed in patients with recurrent nasopharyngeal, sinonasal, and skull base tumors who are at risk of retrograde perineural invasion or dural invasion.
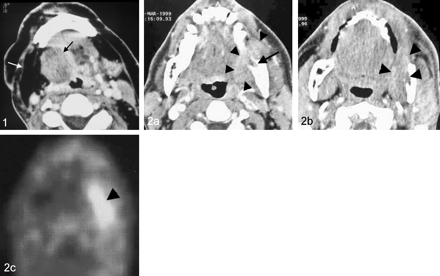
The most commonly used PET radiotracer is FDG. The initial enthusiasm for PET was tempered by limited availability, high cost, and lack of Medicare reimbursement. PET is now widely available in North America, and CMS has confirmed efficacy and now reimburses for PET restaging of HNSCCA. There has been concern of a high false-negative rate for patients treated with radiation therapy if the study is performed within 3 months after the completion of treatment. This has not been our experience, and we have not had problems with false-negative studies performed within 3 months after completion of radiation therapy. We have noticed numerous false-positive studies for treated oral cavity and oropharyngeal tumors. This may be due to artifact from tongue uptake from speech after radiotracer administration, lingual tonsil uptake, retained salivary uptake in the oral cavity, or post-treatment granulation tissue. Similar false-positive findings have also been seen in laryngeal carcinomas treated with combined chemotherapy and radiation therapy and may be due to short time interval (<3 months) after completion of combined chemotherapy and radiation therapy. The diagnostic accuracy of PET to evaluate myocutaneous flap reconstruction for the surgical treatment of advanced tumors has not been adequately evaluated. The metabolic changes that arise from denervation may affect FDG uptake. Further investigations are needed to evaluate this important issue. Despite these limitations, numerous studies have assessed the diagnostic accuracy of FDG PET for detecting recurrent HNSCCA. The data suggest a high sensitivity (71–100%) and moderate specificity (43–100%) (13–24). A review of current literature suggests that FDG PET has a higher diagnostic accuracy as compared with cross-sectional imaging (25, 26) (Fig 2). It must be noted that the most accurate PET results will only be obtained if the information is interpreted in the context of clinical findings as part of a multidisciplinary head and neck oncology team.
Thallium-201 SPECT has been shown to be able to differentiate recurrent tumor from post-treatment changes. Some investigators have suggested that the diagnostic accuracy of thallium-201 SPECT may be superior to CT. The advantages of thallium-201 are that it is readily available and relatively inexpensive compared with PET. Because the brain does not metabolize thallium-201, it may be superior to PET for evaluating recurrent skull base tumors. The primary disadvantage of thallium-201 imaging is the background uptake of thallium-201 by salivary and thyroid glands (14). Normal uptake in the salivary and thyroid glands has the potential to reduce the ability to evaluate oral cavity, oropharynx, larynx, and pyriform sinus tumors accurately. Despite these limitations, the reported diagnostic accuracy of thallium suggests that it be an alternative metabolic technique if PET were unavailable (5, 6, 27, 28).
We are currently in the midst of an evolutionary process that began more than 10 years ago in which metabolic imaging is becoming the accepted technique of differentiating recurrent tumor and post-treatment changes. The role of cross-sectional imaging is to determine the extent of recurrent disease in patients being evaluated for planning of surgical salvage or adjuvant therapy. It is our opinion that the most efficacious imaging paradigm for the evaluation of the postoperative neck is based on the clinical suspicion of disease:
1. No imaging study is warranted for patients treated with early stage disease who are felt to have a low clinical suspicion for recurrent disease.
2. We would recommend PET as the initial study in the following two scenerios: patients treated with advanced disease with low clinical suspicion of recurrence in which imaging may be clinically useful and patients with nonspecific symptoms that could indicate recurrence but without a clinically obvious mass. If the patient has been treated with radiation therapy, the literature suggests that PET should optimally be performed at least 3 months after completion of treatment. In this group, no further imaging would be necessary if the PET study is negative.
3. Cross-sectional imaging should be performed for an equivocal or positive PET study. We also recommend cross-sectional imaging as the initial study in patients with a suspicious palpable mass or biopsy proved recurrence to evaluate the extent of disease for consideration of performing surgical salvage or other adjuvant therapy.
The natural evolution suggests that the imaging technique of choice for evaluating the post-treatment neck will eventually be combined CT-PET systems. Early results suggest that this technique may be superior to CT and PET for detecting recurrent tumor. One must be aware, however, that the CT component of most current CT-PET systems do not have the capabilities of a dedicated CT and therefore have limited image quality. There are currently limitations regarding the ability to angle the gantry, choice of section thickness, and detector technology (one or two detector rows). As a result, we have not substituted the CT performed from the CT-PET for a dedicated diagnostic CT. Once these technical issues are addressed, one can easily envision a time where CT-PET will be the study of choice for evaluating the post-treatment patient.
There is no question that the ultimate “diagnostic accuracy” of any imaging technique is based on the experience of the individual interpreting the study. A thorough understanding of the normal anatomy and the radiologist’s experience with various patterns of recurrence are the most important factors when deciding which technique to recommend (29). We are currently in a continuum where there is growing momentum for metabolic imaging. Members of the head and neck oncology team should base their imaging paradigm for evaluating the post-treatment neck based on institutional expertise and experience with various imaging techniques. If this does not work, another option may well be a combined a CT-MR-PET-SPECT-optical imaging scanner!
Axial contrast-enhanced CT demonstrates a myocutaneous fat flap (white arrow) used for reconstruction following resection of a squamous cell carcinoma of the floor of mouth. The black arrow identifies a minimally enhancing focal soft tissue mass along the deep margin of the flap. Biopsy revealed recurrent tumor.
Fig 2. Fifty-year old man with a left retromolar trigone carcinoma who was treated with combined chemotherapy and radiation therapy as part of a non-surgical organ preservation program.
A, Axial contrast-enhanced CT shows an aggressive soft tissue mass involving the left retromolar trigone (arrowheads). The tumor erodes the anterior aspect of the left mandibular ramus (arrow), which shows progression of the tumor stage to T4.
B, The patient was treated with combined chemotherapy and radiation therapy. CT performed 5 months after the completion of therapy demonstrates an indeterminate minimally enhancing soft tissue mass in the left retromolar trigone. (arrowheads)
C, Axial image from an FDG study demonstrates increased uptake in the treated tumor bed (arrowhead) that was highly suspicious for recurrent tumor. Biopsy revealed squamous cell carcinoma.
Acknowledgments
The authors wish to thank Stephen Gebarski, MD, for his thorough review of the manuscript.
References
- Copyright © American Society of Neuroradiology