Abstract
Summary: Acute vertebrobasilar dissection may cause subarachnoid hemorrhage by rupturing through the adventia or cerebral infarct by progressive occlusion of the true lumen. Recent reports on the endovascular management of this condition have focused on treatment of pseudoaneurysms. We report two cases where angioplasty or stent placement was successfully used to improve compromised blood flow secondary to vertebrobasilar dissection.
Spontaneous dissection of the basilar artery is an uncommon event, although with increased use of MR imaging in acute stroke settings it seems to be recognized more often than in the past (1). Basilar dissection may occur in isolation but more typically develops via antegrade progression of a vertebral artery dissection. Fibromuscular dysplasia and trauma are the most common predisposing factors (2). The clinical course and prognosis are highly variable, ranging from a relatively benign course with complete neurologic recovery to death from brain stem infarction or hemorrhage. In a recent large series of vertebrobasilar dissection, most patients (55%) presented with severe headache or neck pain, and most (84%) had a good or excellent outcome (1). Of the 31 patients in this series, only six underwent surgical or endovascular procedures. In another recent series involving medical treatment alone, however, four of nine patients with intracranial vertebrobasilar dissection suffered permanent disabling deficits (2).
Because many, if not most, patients recover uneventfully, conservative management is often sufficient; however, severe disability or death can result from aneurysmal rupture, distal embolization, or basilar occlusion. In patients with ruptured dissecting aneurysm or symptoms of brain stem ischemia, prompt intervention may be lifesaving.
We report the successful endovascular treatment of two cases of vertebrobasilar dissection by using angioplasty or stent placement. The first patient had acute subarachnoid hemorrhage from spontaneous dissection of the vertebral artery, resulting in intracranial vertebral pseudoaneurysm and antegrade basilar dissection. The second patient presented with symptoms of acute brain stem ischemia secondary to isolated spontaneous basilar dissection.
Case Reports
Case 1
Clinical Presentation.
A 55-year-old woman became acutely unconscious while sitting in her kitchen at home and fell to the floor. She was brought to the emergency room by ambulance and was intubated upon her arrival. Her only medical problem was hypertension. The patient’s Glasgow coma score was 7 in the emergency room. On examination, she briskly localized bilaterally, would not open her eyes, and was intubated. Fundoscopic examination findings were unremarkable. There were no focal cranial nerve or motor deficits discovered on examination. Reflexes were 2+ throughout, and plantar responses were flexor bilaterally. Laboratory tests included cardiac enzymes, electrolytes, and complete blood cell count. The cardiac enzymes were tested because she exhibited S-T elevation on an electrocardiogram. All laboratory values were within normal limits, except for mild hypernatremia (sodium 146 mmol/L) and an elevated white blood cell count (21,100/mm3). Emergent cranial CT demonstrated subarachnoid and intraventricular hemorrhage and hydrocephalus. Blood was seen in all four ventricles.
Emergency cerebral angiography that evening revealed a 3-mm aneurysm of the left posterior communicating artery, intracranial right vertebral artery dissection with tapered narrowing and a visible intimal flap, and a pseudoaneurysm of the right vertebral artery between the posterior inferior cerebellar artery (PICA) and the vertebrobasilar junction (Fig 1A and B). The following afternoon, the patient was transferred to the neurointerventional suite for treatment of the dissecting aneurysm.
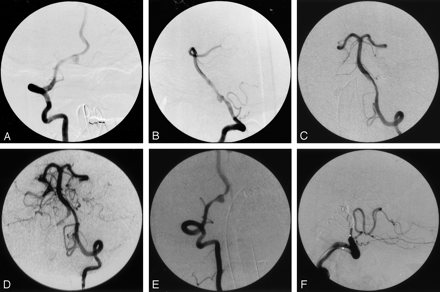
Case 1, a 55-year-old woman with acute subarachnoid hemorrhage.
A, Anteroposterior right vertebral angiogram, showing the eccentric location of the aneurysm relative to the vertebral artery.
B, Lateral right vertebral angiogram, showing aneurysm of the V4 segment of the vertebral artery. Tapered narrowing proximal and distal suggests dissection, which is confirmed by visualization of a linear filling defect representing intima.
C, Sixteen hours later, left vertebral anteroposterior angiogram, showing tapered narrowing of the vertebral from propagation of the dissection, with severe narrowing at the vertebrobasilar junction.
D, Left vertebral angiogram after angioplasty, showing markedly improved caliber from vertebral to basilar artery.
E, Anteroposterior right vertebral angiogram, showing pseudoaneurysm between the right posterior inferior cerebellar artery and the vertebrobasilar junction.
F, Lateral right vertebral angiogram after embolization, showing right vertebral occlusion distal to the PICA.
Intervention.
The patient underwent placement of a right frontal ventriculostomy before undergoing cerebral angiography. She also received fosphenytoin (18 mg/kg phenytoin equivalents) and alpha-aminocaproic acid (10 g once, then 2 g/h thereafter).
The alpha-aminocaproic acid was stopped 2 hours before the procedure. The entire procedure was performed in the neuroangiographic suite under general anesthesia. Bilateral vertebral biplane angiography was first repeated to confirm the initial findings and to obtain motion-free views of the intracranial circulation. Angiography demonstrated an intimal flap of the right vertebral artery extending into the basilar artery, a 4 mm × 6 mm pseudoaneurysm of the right vertebral artery between the PICA and vertebrobasilar junction with tapered luminal narrowing both sides of the pseudoaneurysm, and greater than 70% stenosis of the terminal left vertebral artery (Fig 1C and E). Both vertebral arteries were approximately equal in size.
A 6F guiding catheter (Guider Softip, Target Therapeutics, Fremont, CA) was placed in the left vertebral artery. A 3.5-mm-diameter silicone balloon (Sentry, Target) was advanced over a 0.010-inch guidewire (Transcend, Target) through the region of stenosis such that the proximal end was in the distal vertebral and the distal balloon was in the proximal basilar artery. The balloon was inflated by hand to its nominal diameter of 3.5 mm and was kept inflated for approximately 20 seconds. Two inflations were performed. The postangioplasty left vertebral injection showed less than 40% residual narrowing (Fig 1D).
The guiding catheter was withdrawn and then placed in the right vertebral artery at the C3 level. A microcatheter (Renegade, Target) was advanced into the right vertebral artery beyond the pseudoaneurysm over a 0.014-inch wire (Transcend, Target). After performing an injection to confirm the catheter position, a 2 mm × 4 mm fibered detachable coil (GDC Vortex, Target) was placed in the vertebral artery lumen and across the site of rupture. Three additional 2 mm × 3 mm fibered detachable coils were placed in the proximal and distal lumen and across the aneurysm. The occlusion was completed with two 3 mm × 8 cm detachable coils (GDC-18 Soft, Target). Right vertebral angiography after embolization showed complete distal vertebral occlusion with preservation of the PICA. A repeat left vertebral angiogram showed no filling of the aneurysm and normal filling of the basilar artery with no visible flap. The procedure time was 2 hours 30 minutes. All flush solutions were heparinized (4 U/mL), but no additional heparin was administered, because of the recent aneurysmal rupture.
Postoperative Course.
The patient tolerated the procedure well and was extubated on the fifth hospital day. She remained dependent upon CSF diversion; therefore, a left frontal ventriculoperitoneal shunt was placed on the sixteenth hospital day. Her hospital course was uncomplicated. Neurologically, she remained somnolent throughout her hospitalization and could answer only very simple questions correctly. Her cranial nerve and motor examination remained normal. She was discharged to a nursing home on the twenty-fourth hospital day. She was seen in clinic 8 weeks after the ictus, and she had good functional status. Her only neurologic problems upon examination appeared to be mild perseveration and mild short-term memory loss. She was living at home with her daughter.
Case 2
Clinical Presentation.
A 67-year-old formerly ambulatory man with a history of hypertension, chronic obstructive pulmonary disease, and a prior left middle cerebral artery (MCA) stroke with residual right hemiparesis was transferred from an outside hospital 4 hours after sudden onset of weakness, ataxia, dysarthria, and decreased responsiveness. On initial examination, the patient was mute and quadriplegic but opened his eyes when one spoke to him. Pupils were 3 mm on the right and 2.5 mm on the left and reactive to light. The vertical gaze was fairly intact in his right eye; however, the left eye displayed a severe up-and-down vertical limitation. Horizontal movements were severely affected bilaterally. Initial head CT showed only an old left MCA infarct. The clinical diagnosis of basilar artery thrombosis was made, and the patient was intubated and transferred to the angiographic suite.
Intervention 1.
The procedure was initiated approximately 6 hours postictus. Sedation was provided with 1 mg IV midazolam, and paralysis was achieved with pancuronium at 0.1 mg/kg. Right common carotid angiography showed a weblike stenosis of the internal carotid at the foramen lacerum and a very small posterior communicating artery with minimal filling of the right posterior cerebral artery. The left common carotid injection showed occlusion of the left internal carotid with reconstitution via external carotid anastomoses. A left subclavian injection showed absence of a left vertebral artery (aortogram was not performed). The right vertebral injection showed a distal filling defect of the basilar tip extending into the right P1 segment with occlusion of the right posterior cerebral and left superior cerebellar arteries (Fig 2A). A 6F guiding catheter (Guider Softip, Target) was advanced to the right vertebral artery, and a microcatheter (Renegade, Target) was advanced to the midbasilar artery. Pulsed injections of tissue plasminogen activator (t-PA) (Activase, Genentech, South San Franciso, CA) to a total dose of 30 mg were administered over approximately 90 minutes with no improvement. To attempt mechanical disruption of suspected clot, the guidewire (Transcend, Target) was advanced first into the left and then into the right posterior cerebral arteries, followed by advance of the microcatheter. A selective right posterior cerebral artery injection showed absence of any filling defects. A midbasilar injection after pulling the microcatheter back out of the right posterior cerebral showed much improved distal flow (Fig 2B). At that point, an intimal flap was seen that was retrospectively evident on the initial angiographic runs after careful postprocessing (Fig 2A and C). Because the distal basilar outflow was much improved, we elected to treat the dissection conservatively with systemic heparinization, and the sheath was removed by using a collagen plug (Vasoseal, Datascope, Montvale, NJ).
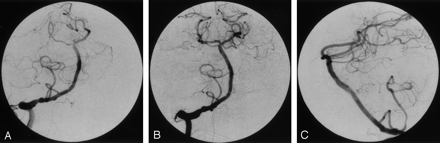
Case 2, a 67-year-old man with acute basilar dissection.
A, Anteroposterior right vertebral angiogram (before superselective catheterization), showing occlusion of the right posterior cerebral and left superior cerebellar arteries with small stumps. This was believed to be secondary to thromboembolism. Irregularity of the intradural right vertebral artery, first thought to represent atherosclerosis, may be secondary to dissection. In retrospect, a small intimal flap was visible in the midbasilar segment on the lateral view.
B, After 30 mg of t-PA, there was no improvement; however, following blind catheterization of the right PCA, the right PCA and left SCA show normalized flow.
C, Lateral right vertebral injection, clearly showing a linear filling defect of the basilar artery indicating dissection. This had been present before intervention but was unrecognized until remasking and pixel shifting were performed on the initial runs.
Intervening Course.
The patient was placed on heparin and remained intubated. The following morning, he was alert, nodding and shaking his head, and pantomiming appropriately with the left side. Strength was intact on the left. He displayed a flaccid hemiplegia on the right. Eye movements improved significantly, though he showed a gaze preference to the left. Over the next few days, he began to regain proximal mobility of his right side and was extubated. Heparin was discontinued 3 days postoperatively, after the patient developed progressive anemia; however, the workup was negative for occult bleeding. On postoperative day 6, he became acutely quadriplegic with small (1-mm) reactive pupils bilaterally. He opened his eyes spontaneously to command but had a fixed gaze deviation to the left. He was initially able to follow commands by shrugging his left shoulder, but he rapidly lost this ability. Airway control was lost, and the patient was emergently intubated. Heparin was once again started, and he was returned to the angiographic suite.
Intervention 2.
On arrival to the neuroangiographic suite the patient was again medicated with IV midazolam and pancuronium. A right vertebral arteriogram showed marked worsening of the basilar dissection with a severely narrowed true lumen, poor distal flow, and extensive thrombus in the false lumen (Fig 3A, B). A 6F guiding catheter (Guider Softip, Target) was advanced into the right vertebral artery. A microcatheter (Renegade, Target) was directed into the basilar by using an 0.014-inch guidewire (Transcend, Target), and the true lumen was cannulated. The exact position of the true lumen was confirmed by a gentle injection within the point of maximal narrowing anterior to the pons. The microcatheter was withdrawn, and the Transcend wire was readvanced along the same course through the true lumen into the right posterior cerebral artery. Using the rapid exchange method, a 3 mm × 13 mm (width × length) coronary stent (Multilink, Guidant Corp, Indianapolis, IN) was advanced and deployed beginning at the proximal entry point of the dissection distally and inflated to 6 atm. The balloon was withdrawn, and a right vertebral injection showed marked improvement but persistent distal narrowing. A second stent, 3 mm × 8 mm (Multilink), was advanced over the wire and placed at the distal basilar, slightly overlapping the first stent. The final right vertebral angiogram showed normal caliber of the basilar artery with dramatically improved filling of bilateral posterior cerebral, superior cerebellar, and anterior inferior cerebellar arteries (Fig 3C and D).
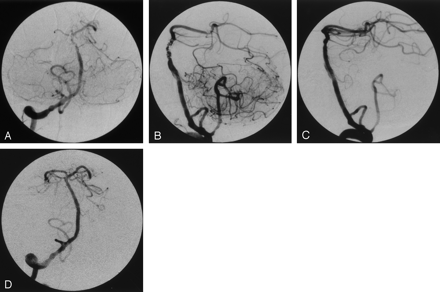
Case 2, 6 days later.
Frontal (A) and lateral right vertebral (B) angiograms. There is worsening of the dissection with multiple branch defects and intraluminal clot. Lateral (C) and anteroposterior right vertebral (D) angiograms after second stent placement. The basilar artery lumen is reestablished, and terminal branches now fill. A small residual of the false lumen is seen anterior to the basilar artery.
Postoperative Course.
Heparin was continued for 24 hours after the procedure, and aspirin and clopidogrel were subsequently started. The morning after the procedure, the patient was again responding appropriately by nodding or shaking his head. He was mildly ataxic, with a left internuclear ophthalmoplegia (INO) and a severe skew deviation with a hypotropic left eye. He also exhibited a combined horizontal and torsional nystagmus. Strength was good on the left, but he displayed a complete right hemiplegia. The patient was not extubated until postoperative day 6, because of concerns about airway protection. MR imaging with MR angiography revealed a subacute left paramedian infarct in the rostral pons and an old left MCA infarct. The left internal carotid artery was occluded, and the left vertebral artery was small or occluded. The basilar artery was visualized and appeared patent. Examination after extubation found him dysarthric and dysphagic. He remained ataxic, with a left INO. He had regained a little movement on his right side, recovered good strength on the left, and is progressing in rehabilitation.
Discussion
There are now many reported cases of endovascular intervention for intracranial vertebrobasilar dissection; all but one case were for treatment of dissecting pseudoaneurysm. To date, the largest series of interventions consists of 18 patients who had endovascular coiling of dissecting vertebral aneurysms by parent vessel occlusion (3).
As reflected in our two cases, the endovascular management of vertebrobasilar dissection depends on the manner of presentation. Patients presenting with mild symptoms may be managed medically with systemic heparinization. Those who present with subarachnoid hemorrhage require urgent treatment because of the high incidence of rebleeding (30–69%), often in the first 24 hours, and the high mortality rate (47%) that follows rebleeding (4). In the setting of basilar dissection with progressive or acute severe neurologic deficits, emergent recanalization and stent placement of the basilar artery may be lifesaving.
In October 1999, Malek et al (5) reported a case of basilar artery stent placement for treatment of an iatrogenic basilar dissection secondary to vertebral angioplasty and stent placement. Basilar dissections presenting with brain stem ischemia pose an altogether different management problem. The diagnosis may be subtle, for both the clinical presentation and angiographic findings may superficially resemble thromboembolism, as in both our case and the case described by Malek et al. The initial finding of a posterior cerebral artery occlusion led them (like us) to suspect thromboembolism and to attempt intraarterial thrombolysis. The improvement after our first intervention probably was effected by passage of a microcatheter altering the morphology of the dissection in favor of the true lumen, rather than a delayed effect of the t-PA. Our customary upper limit for r-TPA in intraarterial thrombolysis is 20 mg, although we are willing to exceed that limit if continuing benefit is demonstrated angiographically or in the basilar artery where failure to recanalize may mean almost certain death. In a report presented a few months before case 2, 14 patients received a mean dose of 25.6 mg intraarterial t-PA. Clinically significant hemorrhage was seen in only the two patients receiving the highest dosage, at dose levels of 50 mg and 68 mg (6). The approved intravenous dose for t-PA in stroke is 0.9 mg/kg to a maximum of 90 mg.
High-resolution biplane angiography in an immobile patient is essential for angiographic diagnosis of basilar dissection, because the only direct evidence may be a faint linear filling defect that can easily be mistaken for a subtraction or streaming artifact. In the vertebral artery, fusiform aneurysms and tapered stenoses are the most common findings. Once a dissection is confirmed, medical therapy may be the most appropriate course of action if all major branches fill and the patient has minimal or no neurologic impairment. If medical therapy fails with the onset of significant or progressing neurologic deficits, stent placement of the affected artery may be the only therapeutic option.
An increasing body of reported cases supports the technical effectiveness of stent placement in treatment of arterial dissection, including the extracranial carotid and vertebral arteries. Stent placement across the injury accomplishes primary repair by exclusion of the inflow zone, with preservation of the parent vessel and a return to normal luminal diameter (7). When to intervene, however, is an unresolved question, because most carotid dissections seem to resolve uneventfully with medical treatment alone (8). Many would agree that stent placement of a carotid dissection is appropriate if ischemic symptoms develop, but treatment of asymptomatic dissections may be unwarranted. On the other hand, the natural course of vertebrobasilar dissection is more variable.
Endovascular intervention of any type in a basilar dissection can be extremely risky, particularly if the false lumen is incorrectly cannulated. To our knowledge, this is only the second reported case of stent placement in a basilar dissection and the first of a spontaneous (ie, not iatrogenic) dissection. Such a procedure should not be attempted until meticulous biplane angiography has delineated the relationship of true and false lumens. If any doubt exists, placement in the true lumen should be confirmed by injection with a microcatheter at the site of proposed deployment before advancing the stent delivery system. In a vessel with critical side branches such as the basilar, it may not be sufficient to know that the distal position of the guidewire is intraluminal, because it is possible to enter a false lumen proximally and reenter the true lumen distally, in which case the false lumen would be stented and side branches would be permanently occluded.
Conclusion
The management of vertebrobasilar dissection must be carefully tailored to its manner of presentation. Ruptured intracranial dissecting aneurysm is associated with high morbidity and mortality in the absence of intervention. The high risk of rebleeding within the first 24 hours calls for aggressive and rapid intervention. In patients who can tolerate sacrifice of the parent vessel, endovascular trapping with fibered detachable coils may offer the best hope of cure, although early results with the reconstructive approach appear promising. Angioplasty alone can decrease vessel narrowing as an adjunct to treatment. To our knowledge, case 2 is the first reported instance of stent placement for a spontaneous basilar artery dissection. The findings of basilar dissection may be subtle; the combination of acute neurologic deficit with basilar branch occlusions may imitate acute thromboembolism. For patients with intractable ischemia from basilar dissection, the use of small flexible coronary stents can reestablish luminal patency and is the only option when medical therapy fails.
References
- Received March 11, 2002.
- Accepted after revision September 6, 2002.
- Copyright © American Society of Neuroradiology