Abstract
BACKGROUND AND PURPOSE: The evolution of apparent diffusion coefficient abnormalities during supratentorial intracranial hemorrhage in normal appearing brain tissue has not been described. Recent investigations using diffusion imaging have revealed increased apparent diffusion coefficient in perihematomal tissue. We report brain tissue abnormalities beyond the visibly abnormal region ipsilateral and contralateral to the hematoma. This preliminary effort should generate meaningful clinical prognostic indicators for moderate size hemorrhages in large scale studies.
METHODS: Using the neurology patient encounter database at a tertiary care hospital, we retrospectively identified patients who presented with acute focal neurologic deficits, had CT scans of the head that confirmed spontaneous intracranial hemorrhage, and had a MR images obtained within the first 6 hr to 30 days postictus. The regions identified as targets of this investigation were the hemorrhage and surrounding T2 signal intensity abnormality and the visibly normal supratentorial cerebral tissue.
RESULTS: Ninety-five patients were admitted during a period of 25 months. Fifteen patients met the criteria for the study. Elevated whole brain diffusion was shown as early as 6 hr after intracranial hemorrhage. This increase in diffusion was comparable in both hemispheres. Diffusion values in the lesion (hematoma plus T2 signal intensity abnormality) increased slowly with peak increases noted 2 to 3 days after the ictus.
CONCLUSION: Diffuse early cerebral response occurs in normal appearing brain tissue both ipsilateral and contralateral to the visibly abnormal hematoma, manifested by increased apparent diffusion coefficient. This response is present before the local response is fully developed. Supratentorial intracranial hemorrhage results in an early diffuse brain response with increased apparent diffusion coefficient in normal appearing brain.
Intracranial hemorrhage (ICH) accounts for 10% to 15% of all strokes and has higher rates of morbidity and mortality than do ischemic strokes of similar volume (1). Spontaneous ICH is associated with a 50% case fatality rate. Despite advances in critical care, the prognosis for survivors of ICH remains dismal. This poor outcome exists, in part, because there is no consensus for the appropriate management of patients with ICH. Unresolved issues ranging from blood pressure management to more aggressive interventions, such as the timing of surgical hematoma evacuation, remain controversial.
One of the main limitations hindering development of interventional protocols for ICH is that detailed knowledge regarding the temporal response of the human brain to ICH at the tissue level is lacking. No test is available to measure this cerebral response over time.
Recently, diffusion-weighted MR imaging has contributed greatly to our understanding of tissue pathophysiology during acute ischemic stroke. The evolution of diffusion-weighted MR imaging abnormalities during ischemia reflects alterations of the tissue ultrastructure and is well described (2, 3). Temporal diffusion-weighted MR imaging changes in patients with ICH, if investigated, may further advance our understanding of the cerebral response to ICH and may show that diffusion-weighted MR imaging has the ability to provide meaningful information regarding the tissue response to an intracerebral hematoma. In this regard, diffusion-weighted MR imaging may prove useful in guiding the management of patients with ICH.
We herein present the first description of the tissue response of the brain to ICH as shown by quantitative diffusion-weighted MR imaging. With this methodology, one can quantify the degree of abnormal diffusion in the entire cerebrum and in selected regions of anatomic interest. We have already described the whole brain diffusion values in normal age-matched control participants. These values are maintained within a narrow range (4). The aims of this study were to describe the diffuse cerebral reaction to ICH by using diffusion-weighted MR imaging, the local tissue response to ICH, and the dynamic nature of these cerebral tissue changes over time.
Methods
Patients
Inclusion Criteria
Using the neurology patient encounter data base at a tertiary care hospital, we retrospectively identified patients who presented with acute focal neurologic deficits, had CT scans of the head that confirmed spontaneous ICH, and had MR images obtained within the first 6 hr to 30 days postictus. All patients underwent diffusion-weighted MR imaging.
Exclusion Criteria
Patients excluded from the study were those with contraindications to MR imaging because of permanent pacemakers or vascular coils, those with CT evidence of hydrocephalus, those with CT evidence of multiple ICH, and those who did not undergo MR imaging during the 30-day time period secondary to medical instability. MR images obtained after surgical evacuation were not included.
Recorded Data
The study cohort was comprised of a nonconsecutive observational case series. For each patient, recorded data included age, sex, date of ictus, time from ictus to all MR imaging (in cases of follow-up studies), blinded neuroradiologic assessment of the causative mechanism and age of the ICH, blood pressure and mean arterial pressure measurements at the first presentation to our facility, blood pressure and mean arterial pressure records closest to the time of the first MR imaging, Rankin score (RS) based on physical and occupational therapy assessments near discharge, and ultimate disposition from the hospital to home, to acute rehabilitation, to a skilled nursing facility, or because of death.
Control Group
The diffusion values quantified from 38 age-matched normal participants and nine patients with ischemic stroke were used as control values. The normal data were from our previous study (4). Whole brain diffusion values are maintained within a tight range during the course of normal adulthood; we were thus able to use this normal subset in our study.
The patients with ischemic stroke who served as control participants were included to represent patients with intracerebral atherosclerotic disease, to ensure that any diffusion changes observed in the patients with ICH were not representative of preexisting cerebrovascular disease and were actually reflective of acute adaptive change.
Imaging Methods
A clinical whole body MR imaging unit (1.5-T GE Signa Echospeed) with a single shot multi-section echo-planar diffusion imaging sequence was used to obtain the data. Imaging protocol included 30 interleaved axial view sections covering the entire brain with a thickness of 5 mm, an image matrix of 128 × 128 zero-filled to 256 × 256, and a field of view of 22 cm. The diffusion gradients were applied in three orthogonal directions, one at a time. Using these three diffusion-weighted images and the image without diffusion gradients, an orientationally invariant diffusion constant map (Dav = Trace[D]/3) was calculated for each pixel. The b value = 1000 s/mm2. Regions of interest were drawn on each section to delineate the lesion. These regions were further analyzed by using distribution analysis. The Dav maps were reduced to 1D by distributing the pixels to 250 bins with a bin width of 2.0 10−7 cm2/s. We fit the diffusion data to a triple gaussian curve for further segmenting out of the tissue from the CSF-filled regions (4). By this method, brain tissue that is not contaminated with CSF can be analyzed. By analyzing the mean of the diffusion constant distribution in the brain tissue, we describe a diffusion value (BDav) that is characteristic of that region of interest. Thus, the parameter BDav represents the mean diffusion in brain regions that were the focus of this analysis.
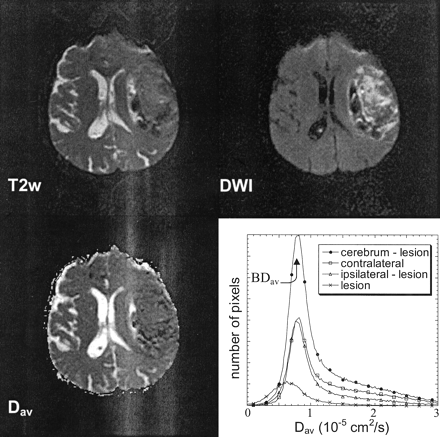
Two regions were identified as targets of this investigation: the hemorrhage and surrounding T2 signal intensity abnormality and the visibly normal supratentorial cerebral tissue. The lesion was defined as the tissue volume containing hemorrhage plus the surrounding area of T2 signal intensity abnormality, indicative of tissue edema. This definition of the lesion was selected because hemorrhage-induced edema can increase lesion volume by as much as 200%, with implications for intracranial pressure elevation, which has a significant impact on patient survival (5). In addition, the removal of all visibly abnormal tissue was necessary to investigate the tissue response in the visibly normal hemispheres and to follow the reaction over time. This process also enabled an estimation of lesion volume and an analysis of diffusion within the lesion. The brain was then separately sampled by regions of interest to make independent analyses of the cerebral reaction ipsilateral to and contralateral to the lesion (Fig 1). A comparison of the whole brain diffusion values was then made between the patients with ICH and the control groups.
Representative example of a typical ICH. T2-weighted image (T2w), diffusion-weighted image (DWI), and diffusion constant image (Dav) are shown. The quantitative analysis that was conducted focused on whole brain diffusion (inclusive of lesion) and brain diffusion ipsilateral and contralateral to the ICH measured by region-of-interest analysis on every section (exclusive of lesion). Lesion analysis included hemorrhage and all T2-weighted surrounding abnormalities that were cored out on every section in which they were visible. Histogram shows the distribution of diffusion constants in each region of interest. Location of the mean diffusion constant (BDav) is shown.
Results
Patient Demographics
During a period of 25 months, 95 patients were admitted with ICH. Twenty-five patients underwent MR imaging within the specified study period. Of these 25 patients, 15 fulfilled our selection criteria. The 15 patients included eight female and seven male patients with an average age of 57 years (age range, 29–87 years).
The time from ictus to MR imaging ranged from 6 hr to 21 days. The mean lesion volume was 72.7 ± 55.5 mL (median, 61.9 mL; range, 16–142.9 mL). Average systolic blood pressure at ictus was 166 ± 38 mm Hg. Minimal systolic blood pressure was 115, with maximal systolic blood pressure at 247 mm Hg. Average mean arterial blood pressure at ictus was 111 ± 28 mm Hg (minimum mean arterial pressure, 77 mm Hg; maximum mean arterial pressure, 177 mm Hg.) Average modified Rankin score was 3.2, with one death occurring in hospital (Table 1).
Patient and participant characteristics
Whole Brain Diffusion over Time
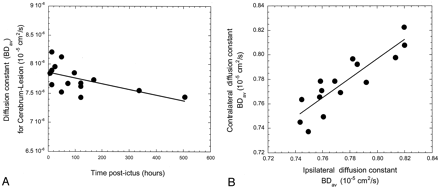
For 15 of 15 patients investigated, quantitative whole brain diffusion-weighted MR imaging revealed increased diffusion in both cerebral hemispheres that persisted for the first 100 hr and was seen as early as 6 hr postictus. The increased diffusion persisted when the brain was measured after removing the visibly abnormal tissue, which eliminates the possibility that the increased diffusion came from edema. Thus, elevated diffusion was observed in normal appearing brain tissue, both ipsilateral and contralateral to the hematoma. The whole brain diffusion approached normal by 500 hr (Fig 2A). The rise in whole brain diffusion correlated positively and significantly with increased systolic blood pressure at ictus (P = .052). However, it did not correlate with mean arterial pressure at ictus, blood pressure or mean arterial pressure at time of MR imaging, or lesion size or composition as defined by the core. Table 1 summarizes the results.
Whole brain diffusion after ICH.
A, Whole brain diffusion is diffusely increased as early as 6 hr after supratentorial ICH.
B, Increased diffusion is comparatively similar in both cerebral hemispheres ipsilateral and contralateral to the ICH.
Ipsilateral Reaction over Time
The increased diffusion noted in the normal appearing white matter ipsilateral to the ICH has an evolution similar to the whole brain diffusion as described above.
Contralateral Reaction over Time
Interestingly, the visibly normal hemisphere contralateral to the core shows evidence of increased diffusion as early as 6 hr, remains constant up to 100 hr postictus, and then approaches normal at 300 hr. The magnitude of the response is approximately the same as in the hemisphere containing blood when the lesion is cored out (Fig 2B)
Lesion Analysis over Time
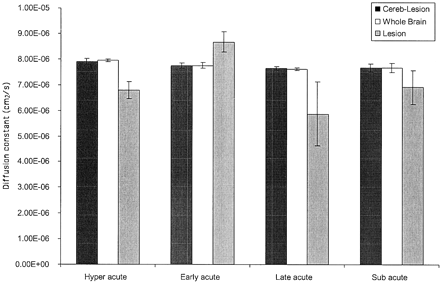
The lesions may be divided into four subgroups based on quantitative diffusion, ictus as defined by history, and MR imaging findings. These were defined as hyperacute (<24 hr), early acute (1–<5 days), late acute (5–<10 days), and subacute (10–21 days). The neuroradiologic assessment was independent of history, and thus two independent assessments (history of ictus and radiologic dating of hemorrhage) were possible.
The diffusion constant measured in the lesion was low during the hyperacute phase (0.68 10−5 cm2/s), increased during the acute phase (0.87 10−5 cm2/s), decreased during the late acute phase (0.59 10−5 cm2/s), and slightly increased during the subacute phase (0.69 10−5 cm2/s) (Fig 3). The diffusion observed in the lesion, therefore, varies with time and correlates with lesion radiologic composition as gauged by MR imaging (edema, composition of hematoma) but not with lesion size or with ictus systolic blood pressure.
Temporal changes during quantitative diffusion. Comparative bar histogram shows the evolution of diffusion changes over time in this series. Whole brain diffusion of the entire group as a whole remains elevated; this effect persists even when the lesion and all surrounding edema are “cored” out. Lesion analysis shows an evolution of diffusion changes. ICH is timed from a combination of history from ictus and neuroradiologic dating. Hyperacute (<24 hr) lesions show a lower diffusion constant because the hematoma is composed of primarily solid cells with unlysed RBCs that rise to a peak during the early acute phase (1–<5 days), with maximum focal edema response. The later phase (5–<10 days) shows its greatest decrease as probably inflammatory mediated cytotoxicity maximizes. The subacute phase (10–21 days) again shows the rise of diffusivity within the lesion as cells lyse and diffusion is no longer restricted.
Discussion
Whole Brain Diffusion over Time
In this study, an increase in whole brain diffusion after spontaneous supratentorial ICH was observed. The abnormally increased diffusion was found in apparently normal brain tissue, as the analysis was performed on both the ipsilateral and contralateral cerebral hemispheres minus all visibly abnormal tissue. In addition, this whole brain response occurred at least as early as 6 hr postictus, which was the earliest MR imaging time point in our series. Remarkably, not only did we observe increased diffusion in the cerebral hemisphere containing the lesion but the elevated diffusion was noted in the entire hemisphere contralateral to the lesion. The magnitude of this not visible but quantifiable response was comparable in both hemispheres (P < .0001).
These data suggest that after spontaneous human supratentorial ICH; a cerebral response evolves that is both early (<6 hr) and diffuse. The elevated diffusion may be a consequence of diffusely increased brain water, a reaction that can be attributed to either an increased cerebrovascular hydrostatic pressure and/or a diffuse cerebral inflammatory reaction with subsequent vasogenic edema. Previous animal and human studies of ICH have shown that blood products are capable of inciting a very early cerebral reaction (5–7). This whole brain response develops earlier than focal cerebral edema, which takes days to reach its peak. In addition, these early tissue changes may explain why moderate volume intracerebral hemorrhages have variable outcomes in comparable patients. Some patients may incite more exuberant hemodynamic and/or inflammatory reactions than others, with greater tissue edema, diffuse cerebral injury, and, as a consequence, worse outcomes. This hypothesis, which requires further investigation, suggests that whole brain quantitative diffusion MR imaging may prove useful for predicting patient outcome after ICH. Although in this series, patient outcome, as measured by Rankin score and by ultimate disposition from the hospital, did not correlate with whole brain diffusion, a study with higher power, using a larger population with serial MR images, might be necessary to show statistically significant outcome prediction.
Increased whole brain diffusion was not observed in the control group with ischemic stroke. Therefore, the increase in whole brain diffusion in patients with ICH was likely temporally related to the inciting acute hemorrhagic event and was not secondary to patient age or baseline arteriosclerosis-related cerebral abnormalities. The increase in whole brain diffusion constant (BDav) did not correlate with lesion size. However, it did correlate positively and significantly with the systolic blood pressure at ictus. This suggests that the increased diffusion may be reflective of a systemic reaction, a possible consequence of acute intracranial pressure elevation whereby the body attempts to raise blood pressure, to sustain a new steady state of cerebral perfusion. This likely has a significant effect on at least local tissue pressure. Therefore, the absence of T2 intense cerebral edema and/or measurable intracranial pressure elevation in patients with ICH does not always indicate normal regional tissue pressure; this has been illustrated by previous reports of cerebral herniation without measurably increased intracranial pressure (8).
Lesion Analysis over Time
The changes in core diffusion over time are reflective of tissue composition at the time the diffusion-weighted MR imaging and/or MR imaging was performed. Fluctuation of core diffusion may be divided arbitrarily into four groups, based on the time from ictus to MR imaging, and defined as hyperacute (<24 hr), early acute (1–<5 days), late acute (5–<10 days), and subacute (10–21 days). The diffusion of the hyperacute lesion is low, and radiologic MR imaging characterization of the core revealed a composition of solid RBCs containing mostly oxy- or deoxyhemoglobin. The sensitivity of MR imaging to RBC oxidative changes has been shown previously in animal studies of ICH (9). The hyperacute core, therefore, is hypercellular and lacks freely diffusing cells. Presumably, this results in the observed low quantitative diffusion. An analogous situation has been described in studies of patients with hypercellular malignant tumors with which the boundary between low and high diffusion predicts the margin of cellular infiltration and tumor edema (10). The magnitude of the decreased diffusion in the ICH core is not as great as in cases of ischemic stroke. The extremely low value seen in cytotoxicity-induced sodium-potassium ATP-dependent pump failure in cases of ischemic stroke is not observed, and thus, the lower diffusion characterizing the hyperacute ICH core is unlikely related to ischemia.
During the early acute stage, when clinical deterioration and evidence of cerebral edema shown by CT have been described as occurring from days 2 to 5 in other studies (11), cell lysis and reactive edema have blossomed in the lesion and diffusion is elevated. This elevated diffusion gradually falls and approaches normal to low during the late acute and the subacute stages when an intense neutrophil and then macrophage inflammatory response infiltrates the core. The hypercellularity of this inflammatory infiltrate or the cytotoxicity that ensues causes the ICH core diffusion to be low. Thus, the lesion diffusion peaks later than the whole brain diffusion, because it takes more time to fully evolve, with a different rate of rise and fall.
Our study did not reveal any evidence of perilesional ischemia (reduced diffusion) in the region surrounding the hemorrhage, the ipsilateral hemisphere, or the entire cerebrum. These findings are in contrast to those of several animal models of ICH that display variable degrees of ischemia or compromised cerebral perfusion (12–16). On the other hand, a recent human study did not show ischemia in most of the cases that it reported (17). This apparent difference between animal models and human studies may be explained in that most animal models of cerebral responses to ICH are studied from ictus, whereas human ICH cannot be studied at this point. The data on the human cerebral response to ICH during this very early postictus phase stems from autopsy studies (18) that exhibit perihematomal petechial satellite hemorrhages but no rim of ischemic tissue.
During the first hours postictus, outcome is unpredictable. Primary hematoma expansion evolves within 3 hr, and maximal blood pressure rise occurs (19), primarily as an acute compensatory response to maintain cerebral perfusion pressure. Perhaps the failure of hyperacute ICH evacuation was a consequence of a natural rebleeding diathesis, by failing to self-tamponade the expanding hematoma, and a failure to achieve steady state between acutely elevated intracranial pressure and cerebral perfusion pressure (20). By 4 to 6 hr postictus, surviving patients probably attain homeostasis, and most animal (21) and human studies of this phase do not show evidence of ongoing perilesional ischemia (17, 22, 23). By this time, our study found diffusely increased whole brain diffusion in the absence of a maximally matured focal response. This diffuse brain reaction, therefore, may not be maladaptive; it might play a significant role in achieving this new steady state.
In the 48 to 72 hr postictus, irritative blood products induce cytotoxic edema, and the focal intracerebral mass effect becomes fully developed. In animal models, the cerebral tissue reaction to a hematoma, as opposed to an infused saline mass, is different, with significantly more cytotoxicity to intracerebral blood products, especially to thrombin (24). In addition, for patients with thrombolytically induced ICH, focal cytotoxic edema does not develop to the same extent as it does with spontaneous ICH (25). During this phase, core diffusion peaks, as mentioned above, and whole brain diffusion is already high. Although this edema eventually abates, the mortality rate associated with this phase, secondary to cerebral herniation, is unfortunately known to be very high. Moreover, the morbidity rate for survivors of this phase is also significant. Post-ICH cognitive and motor deficits may be explained by pathologic findings evident at autopsy, including myelin pallor, cavitation, and diffuse white matter damage in the perihematomal region. ICH surgery trials that have used thrombolytics to aid in hematoma evacuation or that have effectively removed blood products, both in animal and human studies, have shown better outcomes, presumably by removing these cytotoxic blood products.
Conclusion
Temporal evolution of diffusion after spontaneous supratentorial ICH shows that postictus, an early global brain response occurs. We speculate that this may be the result of elevated systemic hydrostatic pressure to maintain cerebral perfusion pressure and to tamponade the hemorrhage. This response is probably necessary for hyperacute survival after spontaneous ICH. However, as focal cytotoxic edema develops in response to free interstitial blood products, steady state is lost as volume rises and intracranial pressure increases. In an attempt to maintain homeostasis, the brain enters a vicious cycle of increased tissue hydrostatic pressure as a consequence of high intracranial pressure to maintain cerebral perfusion pressure. At a critical point, compensation is no longer viable because of the rigidity of the cranium, and cerebral herniation occurs. Our data set is fairly representative of patients who have medium to large hemorrhages that pose the most clinical dilemmas as they evolve. Patients with cataclysmic presentations who die as a result of immediately fatal hemorrhages before undergoing MR imaging are not included in this data set. In addition, patients who underwent surgical evacuation before undergoing their first MR imaging session were excluded, because surgery may abolish the capacity to study the temporal evolution of the cerebral response by removing both mass effect and cytotoxic blood products. Our analysis was restricted to supratentorial ICH, because quantitative diffusion analysis is limited to the cerebral hemispheres, brain stem and cerebellar hemorrhages do not allow for comparison with the contralateral side, and normal diffusion measurements in this region are not yet well defined. Thus, the above evolution of diffusion changes does not apply to infratentorial ICH. We also excluded patients with radiologic evidence of hydrocephalus because hydrocephalus may cause transudation of CSF into the interstitium and confound a rise in whole brain diffusion (26, 27). In summary, this study represents the first effort to quantify the temporal changes of diffusion after spontaneous ICH. These changes suggest a hyperacute development of whole brain edema and acute focal brain response, show a correlation between core diffusion and lesion composition, and provide an opportunity to study brain ultrastructure in a noninvasive fashion.
References
- Received September 24, 2002.
- Accepted after revision November 22, 2002.
- Copyright © American Society of Neuroradiology