Abstract
BACKGROUND AND PURPOSE: The best strategy for treatment of subarachnoid hemorrhage due to ruptured cerebral aneurysm is obliteration of the aneurysm as soon as possible. Early surgery is desirable if the patient does not develop severe vasospasm or is clinically stable. However, if the patient has already developed severe vasospasm on admission, surgery may carry the risk of increasing the severity. We evaluated the safety and effectiveness of combined Guglielmi detachable coil (GDC) embolization and angioplasty in a single session for the treatment of ruptured aneurysms associated with symptomatic vasospasm.
METHODS: From January 1992 to January 2001, 12 consecutive patients with ruptured aneurysms associated with symptomatic vasospasm were treated. Patients were classified as Hunt and Hess grade 2 (n = 1), 3 (n = 6), 4 (n = 4), or 5 (n = 1) and Fisher CT group 2 (n = 1), 3 (n = 10), or 4 (n = 1). They underwent GDC aneurysm occlusion and balloon angioplasty (n = 6), intraarterial papaverine infusion (n = 2), or both (n = 4) in a single session. In nine patients, aneurysm coil occlusion was performed first.
RESULTS: Complete GDC occlusion was achieved in eight patients, a small neck remnant persisted in three, and embolization was incomplete in one patient. In all patients, angiographic improvement of vasospasm was obtained. In one patient, a thromboembolic complication occurred and was treated with urokinase. Clinical outcomes at discharge were good recovery in six, moderate disability in two, severe disability in three, or death in one.
CONCLUSION: Endovascular treatment can be the first therapeutic option for ruptured aneurysms associated with severe vasospasm on admission. It offers some advantages over surgery in this setting, but these are balanced by the risk of thromboembolism.
During the past decade, early surgery for ruptured intracranial aneurysms has gained favor (1–10). However, some patients may not be evaluated for surgery until several days after subarachnoid hemorrhage, for various reasons. In these late patients, if ischemic deficit from vasospasm develops or is impending, immediate surgery typically is avoided (3–5, 8, 11). As a consequence, these patients remain at risk for rehemorrhage and often progress to debilitating stroke, as aggressive hypervolemic hypertensive treatment typically is not pursued. Recently, the rationale for delayed surgery for patients with ruptured aneurysm and symptomatic vasospasm has been questioned (5, 9).
In this group of patients, endovascular treatment may offer advantages compared with surgery. We evaluated the safety and effectiveness of single-session Guglielmi detachable coil (GDC; Target Therapeutics/Boston Scientific, Fremont, CA) aneurysm occlusion and balloon angioplasty and/or intraarterial papaverine infusion in patients with unsecured ruptured aneurysms and symptomatic vasospasm.
Methods
Descriptions of Patients and Aneurysms
From January 1992 to January 2001, 12 consecutive patients with ruptured intracranial aneurysms associated with symptomatic vasospasm were treated at the University of California at Los Angeles Medical Center. All patients were transferred from another hospital. The reasons for transfer were anticipated surgical difficulty with cerebral vasospasm in nine and failed surgery (attempted clipping but unable to clip the aneurysm because of brain swelling) in three. The clinical and aneurysm characteristics of these patients are listed in Table 1.
Clinical characteristics of 12 patients treated endovascularly during a single procedure for ruptured intracranial aneurysm and symptomatic vasospasm
Seven aneurysms (58%) were located in the anterior circulation, and five (42%) were in the posterior circulation. All aneurysms were small (4–10 mm in the largest diameter). One aneurysm (8%) had a wide neck (>4 mm), and 11 (92%) had a small neck (4 mm).
When the patients were initially admitted to the hospital, one patient was classified as Hunt and Hess grade 2; six patients, grade 3; four patients, grade 4, and one patient, grade 5. On the basis of the amount and distribution of subarachnoid hemorrhage at CT, one patient was classified in Fisher group 2, 10 patients were in group 3, and one patient was in group 4.
Symptomatic vasospasm was suspected because of a downhill clinical course as evaluated by the referring physician. In patients classified as Hunt and Hess grades 4 and 5, symptomatic vasospasm was suspected by using transcranial Doppler sonographic evaluation. Admission to our institution ranged from 2 to 17 days after initial bleeding. On the basis of angiographic findings and clinical assessment, the therapeutic alternatives were discussed between the vascular neurosurgery and interventional neuroradiology teams.
Treatment
With the patients under general anesthesia and with use of systemic anticoagulation with intravenous heparin, these patients underwent both GDC aneurysm occlusion and endovascular treatment of vasospasm with balloon angioplasty and/or papaverine HCl (Bedford Laboratories, Bedford, OH) infusion by using standard endovascular techniques (12–17). After the procedure, systemic heparinization was reversed with intravenous protamine sulfate, except in patients with wide-necked aneurysms; for wide-necked aneurysms, systemic anticoagulation was continued for 24 hours to decrease the risk of thromboembolic complication. After the patients were returned to the neurosurgical intensive care unit, standard medical management for vasospasm was continued, including calcium channel blockers, hypervolemic therapy, and intensive monitoring with bedside transcranial Doppler sonography and cerebral blood perfusion studies.
When vasospasm of the parent artery was not extremely severe, GDC aneurysm occlusion was performed before balloon angioplasty. Subsequently, mechanical dilation with balloon angioplasty for proximal vasospasm was performed and augmented with intraarterial papaverine infusion for distal-vessel vasospasm. Angioplasty was usually performed segmentally from distal to proximal.
Papaverine infusion was also performed for proximal vasospasm if balloon angioplasty was technically not feasible. One hundred to 300 mg of papaverine was slowly infused over 30 minutes (5 mg/min). When severe vasospasm was present in the proximal portion of parent artery of the aneurysm, mechanical and/or chemical angioplasty was performed before GDC aneurysm occlusion.
Angiographic Obliteration, Vasospasm, and Follow-up
The rate of angiographic obliteration was evaluated by immediate postembolization cerebral angiography. The anatomic aneurysm occlusion results were classified as complete occlusion (no contrast material filling of the aneurysm dome, body, or neck), small neck remnant (residual filling of part of the aneurysm neck), and incomplete occlusion (some contrast material filling of the dome due to incomplete GDC packing).
Vasospasm with greater than 50% narrowing (estimated original caliber) was categorized as localized or diffuse. Localized vasospasm was defined as vasospasm with greater than 50% narrowing only in the vascular territory of the ruptured aneurysm. Diffuse vasospasm was defined as vasospasm with greater than 50% narrowing in the vascular territory of the ruptured aneurysm and in at least one other vascular territory (eg, right anterior circulation involved in addition to vertebrobasilar territory after basilar tip aneurysm rupture).
Follow-up cerebral angiography was usually performed 5–14 days after GDC aneurysm occlusion. Clinical assessment was performed at discharge from the hospital. The best level of function was graded according to the Glasgow Outcome Score (18).
Results
Overall Findings
GDC aneurysm occlusion was technically feasible in all 12 patients. Complete GDC aneurysm occlusion was achieved in eight patients (67%). A small neck remnant persisted in three patients (25%), and embolization was incomplete in one patient (8%) (Table 2).
Results of GDC aneurysm occlusion, endovascular treatment of vasospasm, and clinical outcome
GDC aneurysm occlusion was performed first in nine patients. For these patients, after aneurysm treatment, balloon angioplasty was performed in six, intraarterial papaverine infusion in one, and both balloon angioplasty and intraarterial papaverine infusion in two (Table 2). In three patients with severe vasospasm, chemical and/or mechanical angioplasty was performed before GDC aneurysm occlusion. All patients had angiographic improvement of vasospasm after endovascular treatment.
One thromboembolic complication occurred during embolization of a left middle cerebral artery aneurysm. After GDC aneurysm occlusion, immediate urokinase infusion and angioplasty resulted in complete revascularization of a middle cerebral cortical branch. This patient did not experience new neurologic deficit after the procedure and thrombolysis. Balloon angioplasty of the A1 segment of the anterior cerebral artery technically failed in three patients.
The relationship between Hunt and Hess grade and clinical outcome is shown in Table 3. Six patients (50%) patients had good recovery, two (17%) were moderately disabled, three (25%) were severely disabled, and one patient (8%) died. In the patients with a poor outcome, two showed diffuse bilateral distal vasospasm treated with papaverine and one was transferred with severe meningitis from a ventricular shunt infection. One patient, classified as Hunt and Hess grade 5, died despite angiographic improvement.
Hunt and Hess grade and clinical outcome
The relationship between vasospasm degree or distribution and clinical outcome is shown in Table 4. Patients with diffuse angiographic vasospasm with similar severity vasospasm in a different vascular territory away from the ruptured aneurysm faired worse after treatment, with only three of eight having good recovery. Three of four patients with less severe, localized vasospasm confined to the vascular territory of the aneurysm had good recovery.
Vasospasm type compared with clinical outcome
Illustrative Cases
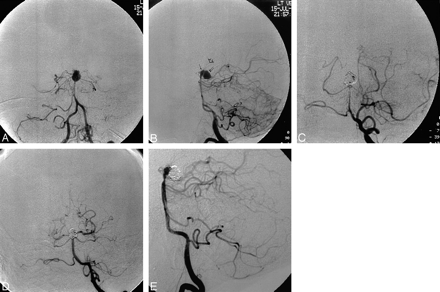
Patient 6 was a 53-year-old woman with a subarachnoid hemorrhage from a ruptured basilar tip aneurysm. Fourteen days after hemorrhage, she was transferred to our hospital for a higher level of care. On examination, she was obtunded, was unable to follow simple commands, and had left hemiparesis. Cerebral angiography revealed a 6-mm basilar tip aneurysm and diffuse severe bilateral anterior and posterior circulation vasospasm (Fig 1A and B). Balloon angioplasty was performed of both supraclinoid internal carotid arteries and M1 segments of the middle cerebral arteries. Direct intraarterial papaverine (100 mg) infusion of the right A1 origin was performed as well. Aneurysmography demonstrated perforators arising from the tip; therefore, the goal of aneurysm treatment was to obliterate the posterior sac of the aneurysm, which was executed without incident (Fig 1C). The diffusely narrowed basilar artery was then opened with balloon angioplasty (Fig 1D and E). Follow-up angiography showed patency of both posterior cerebral arteries and depicted thalamoperforators as well as improved vessel caliber of vessel segments after angioplasty. The patient eventually required a ventriculoperitoneal shunt. Before discharge to a rehabilitation center, she was alert and oriented, was following commands, and had improved motor strength (5/5 right upper and lower extremities, and 4/5 and 3/5 left lower and upper extremities, respectively).
Patient 6, a 53-year-old woman with a ruptured basilar tip aneurysm 14 days after initial hemorrhage.
A and B, Left vertebral artery angiograms, frontal (A) and lateral (B) projections, show severe vasospasm of the basilar artery and posterior cerebral arteries. Present is a basilar tip (single solid arrow) and aneurysm (double solid arrows) complex pointing posteriorly. Note thalamoperforators (open arrow) appearing to arise from the basilar tip.
C, Left vertebral artery angiogram, frontal projection, shows partial coiling of the aneurysm.
D and E, Left vertebral artery angiograms, frontal (D) and lateral (E) projections obtained after embolization of the aneurysm and balloon angioplasty, show improved caliber of the midbasilar artery. Note residual vasospasm still exists at the origin of aneurysm entry zone.
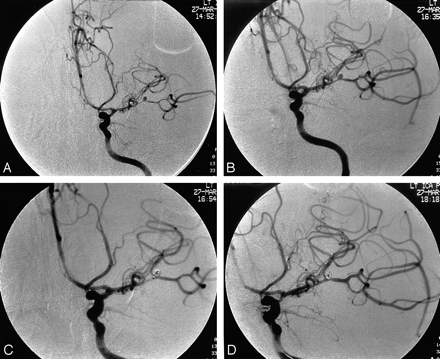
Patient 8 was a 32-year-old man with a severe headache and decreased level of consciousness from a subarachnoid hemorrhage. At another hospital, cerebral angiography suggested a small 2-mm left middle cerebral bifurcation aneurysm. He was taken to surgery there, but no aneurysm was identified during exploration. He was transferred to our hospital on posthemorrhage day 6 after neurologic worsening with lethargy and mild aphasia. At repeat cerebral angiography, the left middle cerebral artery aneurysm was identified as was isolated moderate and severe left anterior circulation vasospasm, worst in the left M1 segment of the middle cerebral artery (Fig 2A). Intraarterial papaverine (300 mg total) infusion of the left supraclinoid carotid and M1 segment of the middle cerebral artery was performed, resulting in mild increase in vessel caliber (Fig 2B). The 2-mm middle cerebral artery bifurcation aneurysm was then embolized with 2-mm GDC-10 coils, with multiple coil repositionings performed before stable coil position was obtained (Fig 2C). At selective postembolization angiography, a thrombus was identified in the prefrontal branch of the middle cerebral artery. The origin of the prefrontal branch of the middle cerebral artery was selectively catheterized, and 25,000 U of intraarterial urokinase was infused followed by 200 μg of intraarterial nitroglycerin, which resulted in opening of the vessel segment. The M1 segment of the middle cerebral artery then became restenosed. The M1 segment vasospasm was then retreated with 150 mg of intraarterial papaverine with no substantial improvement; therefore, balloon angioplasty of the M1 segment was performed, which opened the vessel segment (Fig 2D). The patient’s mild aphasia eventually improved.
Patient 8, a 32-year-old man with a 2-mm left middle cerebral bifurcation aneurysm 6 days after rupture.
A, Left internal carotid artery angiogram, frontal projection, shows the aneurysm as well as focal severe vasospasm of the left M1 and M2 segments of the middle cerebral artery.
B, After intraarterial papaverine infusion in the left anterior circulation, the left M1 and M2 segments have increased in caliber.
C, A microcatheter is positioned at the base of the aneurysm after careful positioning of a 2-mm GDC-10 coil within the aneurysm.
D, Left internal carotid artery angiogram obtained after embolization, intraarterial thrombolysis, and balloon angioplasty shows improved caliber of treated vessel segments and open prefrontal middle cerebral artery branch.
Discussion
In this study, we analyzed our experience in the treatment of 12 patients with unsecured aneurysms and symptomatic vasospasm. These patients were not considered surgical candidates because of severe symptomatic vasospasm; therefore, their aneurysms were treated with GDC occlusion, and vasospasm was treated with balloon angioplasty and/or papaverine infusion as part of a single procedure. GDC aneurysm occlusion and endovascular treatment of vasospasm was technically feasible in all patients. For patients classified as Hunt and Hess grades 2 and 3, five of seven patients experienced good recovery at discharge. Of those classified as Hunt and Hess grades 4 and 5, only one of five patients experienced good recovery. Patients with more severe diffuse angiographic vasospasm with similar severity vasospasm in a different vascular territory away from the ruptured aneurysm faired worse after treatment, with only three of eight having good recovery. Three of four patients with less severe, localized vasospasm confined to the vascular territory of the aneurysm had good recovery.
Preoperative cerebral ischemia has been viewed as a relative contraindication for immediate surgery for patients with ruptured intracranial aneurysms. However, as limited reported surgical experience exists on the treatment of these patients, no recommendation has been established. Aside from anecdotal descriptions, results of endovascular treatment for these patients are also scarce (19). Solomon et al (9) reported that two of three patients with signs of progressive delayed ischemia before surgery 4–7 days after initial hemorrhage had good or excellent outcome, but details of these cases were not provided. As an alternative to delayed surgery in these patients, based on a series of five patients, Le Roux et al (5) recommend urgent surgical obliteration of selected ruptured aneurysms followed by immediate postoperative balloon angioplasty for symptomatic vasospasm. Four of five patients in their study were reported to have sustained moderate to good clinical improvement at 6-month follow-up. They suspect, based on animal experiments and their clinical experience, that the poor results and ischemic events that surgeons have observed after operating in the presence of vasospasm may result from other factors such as hypotension, dehydration, or hypoxia encountered during surgery (5).
Compared with surgery, endovascular therapy does not appear to have an unfavorable effect on the occurrence of symptomatic vasospasm (20). In patients with unsecured aneurysms and symptomatic vasospasm, endovascular treatment may offer advantages compared with surgery. In addition to its less invasive aspects, endovascular treatment offers time advantages, as both ruptured aneurysm and severe vasospasm can be treated during a single procedure. Symptomatic vasospasm can be treated earlier, which may improve clinical outcome (12, 13, 21–23). Furthermore, brain manipulation can be avoided. Vasospasm in adjacent vessels in certain aneurysm locations, such as the posterior circulation, can complicate surgery as ischemic brain may be retracted. Surgical manipulation and temporary clipping of spastic cerebral blood vessels may indeed worsen arterial narrowing, precipitating delayed ischemic deficit. Partial securing of difficult-to-approach or wide-necked aneurysms may allow some protection from rebleeding to allow hypervolemic hypertensive therapy and possible delayed surgery. However, these advantages are tempered by the disadvantages of endovascular therapy: increased risk of thromboembolic complication and questioned long-term permanence of GDC aneurysm treatment (15). If a thromboembolic event occurs, intraarterial thrombolysis with tissue plasminogen activator (urokinase no longer available) carries substantial risk of catastrophic aneurysm rebleeding, in addition to the risk of intraparenchymal hemorrhage, even if the ruptured aneurysm has been filled with coils.
A basic technical strategy is immediate embolization of the aneurysm to prevent secondary rupture, followed by mechanical and/or chemical angioplasty. However, there are two possible scenarios for initial angioplasty before embolization. First, when the parent artery of the aneurysm is already occluded, it is necessary to open the parent artery to access to the aneurysm. Aneurysm embolization in occlusive conditions may suggest a thromboembolic complication, and may necessitate thrombolysis of the unprotected aneurysm. One thromboembolic complication occurred in this series when the microcatheter was obstructed during GDC delivery, resulting in thrombus formation around the microcatheter.
Second, when the patient has already developed focal severe neurologic symptoms (eg, hemiparesis), vasospasm of the representative artery should be treated first. In patient 6 in our series, the ruptured aneurysm was located in the basilar tip with severe basilar artery and bilateral internal carotid artery vasospasm. The patient demonstrated hemiplesia; therefore, internal carotid artery angioplasty was performed first, before aneurysm embolization.
Balloon angioplasty is the first choice when vasospasm exists in proximal vessels such as the intracranial internal carotid artery, M1 segment, A1 segment, vertebral artery, basilar artery, and P1 segment. If vasospasm exists in the more distal portion of the circle of Willis, papaverine infusion must be performed.
Another important technical challenge is treatment of the A1 segment of vasospasm. Anatomically, the angle of the A1 segment and distal internal carotid artery is usually sharp (<90 degrees). Therefore, mechanical angioplasty with a microballoon is generally challenging. Eskridge and Song (12) reported technical consideration of A1 angioplasty with good technical success. To achieve good anchoring of the balloon, the microguidewire must be far advanced in the distal A2 segment. When both A1 and M1 segments show vasospasm (which is a common feature), chemical (papaverine) angioplasty of the A1 segment should be attempted first. If mechanical or chemical angioplasty of the M1 segment is successfully performed, subsequent infused papaverine tends to be diffused away into the middle cerebral artery territory because of increased blood flow. In this situation, Eskridge and Song suggest temporal balloon occlusion to maximize blood flow and papaverine into the anterior cerebral artery. More flexible and shorter (8–12 mm) microballoons will be necessary for safe and successful angioplasty of the A1 segment.
Conclusions
On the basis of our preliminary results, endovascular treatment appears to be a reasonable therapeutic option for patients with ruptured aneurysms and symptomatic vasospasm. Endovascular treatment may improve the clinical outcome for these selected patients compared with that of current treatment by allowing earlier aggressive endovascular treatment of symptomatic vasospasm. However, patients classified as Hunt and Hess grades 4 and 5, who have more severe diffuse vasospasm, including involvement of other vascular territories, continue to have a poor prognosis despite treatment. Although more experience is needed, outcome comparison between immediate surgery followed by treatment of vasospasm and single-session endovascular treatment for both the unsecured aneurysm and symptomatic vasospasm may be helpful in establishing a recommended management approach.
References
- Received February 20, 2002.
- Accepted after revision August 10, 2002.
- Copyright © American Society of Neuroradiology