Abstract
Summary: Carotid T occlusion (intracranial carotid bifurcation occlusion with involvement of A1 and M1 segments) is associated with poor outcome. In most cases, treatment with intraarterial thrombolysis within a 6-hour window has been unsuccessful. We describe the case of a 26-year-old woman who presented with severe neurologic deficits (National Institutes of Health Stroke Scale score of 23) secondary to angiographically proved right carotid T occlusion. She was treated with intraarterial infusion of recombinant tissue plasminogen activator that was started less than 3 hours after symptom onset (26 mg administered during 2 hours 15 minutes). Thrombolysis resulted in recanalization of all major intracranial vessels and complete neurologic recovery. Early intraarterial thrombolysis may be effective in the treatment of patients with carotid T occlusion and should be considered for appropriate candidates.
Acute occlusion of the intracranial internal carotid artery (ICA) at the level of its bifurcation (carotid T) is a catastrophic event that usually results in massive cerebral swelling and grim clinical outcome (1, 2). Although IV administered thrombolysis constitutes the standard of care for the acute treatment of ischemic stroke, recanalization rates are lower when the occlusion affects larger vessels (3). The intraarterial route could be superior in those cases by allowing a more effective delivery of the lytic agent into the large clots. Intraarterial thrombolysis can improve clinical outcome of patients with middle cerebral artery (MCA) occlusions treated within 6 hours of stroke onset (4). However, the treatment of carotid T occlusion with intraarterial thrombolysis has been mostly disappointing in the past (5–8).
We report the case of a young woman who presented with severe right hemispheric ischemia secondary to carotid T occlusion and was successfully treated with intraarterial recombinant tissue plasminogen activator (rtPA) thrombolysis that was started less than 3 hours after symptom onset.
Case Report
A 26-year-old previously healthy woman presented to our emergency department with acute-onset left-sided weakness and dysarthria. Her symptoms began suddenly while she was teaching a class and worsened within minutes. She did not complain of any major headache or neck pain and had no nausea, vomiting, or transient loss of consciousness.
The patient had no history of smoking, alcohol use, or recreational drug use. Her family history was negative for stroke, neurologic disorders, and vascular risk factors. Her only regular medication was an oral contraceptive pill with low estrogen content.
She arrived at the hospital 75 minutes after symptom onset. Her pulse was 84 beats per minute and regular, and her blood pressure was 140/82 mm Hg. Body habitus was unremarkable. No carotid bruits or cardiac murmurs were audible. The patient was drowsy and kept her eyes closed. Once aroused, either by repeated calls or rather vigorous physical stimulation, her answers were appropriate but slow. Right gaze deviation and dense left homonymous hemianopia were noted. Her speech was markedly dysarthric. There was a left flaccid hemiplegia equally involving face, arm, and leg associated with extensor plantar response, hypesthesia, and neglect on the same side. The National Institutes of Health Stroke Scale score was 23. The results of routine blood work and ECG were unremarkable. CT of the head showed a hyperattenuated MCA sign on the right side, with probable subtle effacement of the right sylvian fissure. It was recommended to the patient and her family to proceed with cerebral angiography and intraarterial thrombolysis if technically amenable.
Angiographic and Endovascular Procedure
The procedure, goals, rationale, treatment alternatives, and risks of angiography and intraarterial thrombolysis were thoroughly discussed with the patient and her spouse. All of their questions were answered, and they expressed their desire to proceed.
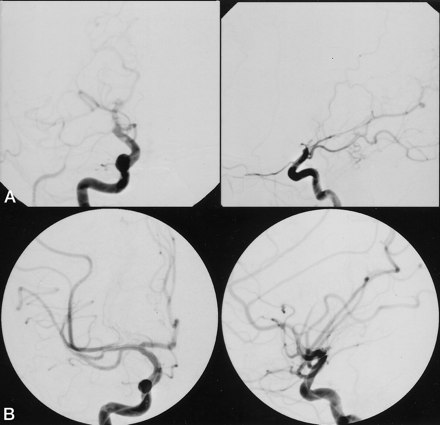
Because of the patient’s inability to maintain a stationary head position, the procedure was performed with the patient under general anesthesia. Using a modified Seldinger technique, a 6F arterial sheath was placed in the right femoral artery and a 6F guide catheter (Target Therapeutics, Inc., Fremont, CA) was advanced into the brachiocephalic arteries. Digital subtraction angiography of the right carotid bifurcation and both ICA and intracranial circulations were obtained. The digital subtraction angiograms showed occlusion of the supraclinoid right ICA distal to the origins of a large posterior communicating artery and the anterior choroidal artery. On the angiograms of the left carotid artery, opacification of the right A2 segment via the anterior communicating artery but no cross filling of the right MCA circulation secondary to a thrombus in the right A1 segment was observed. There were minimal pial collaterals to the distal right MCA circulation from the right posterior cerebral artery and anterior cerebral artery circulations (Fig 1A). There was no evidence of a right carotid dis-section. With the guide catheter in the cervical right ICA, an Excel 14 microcatheter (Target Therapeutics) was advanced over a Transend 14 microguidewire (Target Therapeutics) into the anterior and superior right M2 branch. A digital subtraction angiography series obtained after gentle hand injection of the microcatheter showed occlusion of the anterior and superior M2 branch. The microcatheter was then advanced over the microguidewire into the posterior and inferior right M2 branch. A digital subtraction angiography series obtained at this location showed occlusion of the posterior and inferior M2 branch.
Angiograms from the case of a 26-year-old previously healthy woman who presented to the emergency department with acute onset of left-sided weakness and dysarthria.
A, Cerebral angiogram (anteroposterior [left] and lateral views [right]), obtained after injection of the right ICA and before thrombolysis, shows complete occlusion of the supraclinoid right ICA distal to the anterior choroidal artery. There is no opacification of the A1 and M1 segments due to extension of the thrombus into those segments.
B, Cerebral angiogram (anteroposterior [left] and lateral [right] views), obtained after injection of the right ICA and after intraarterial thrombolysis with 26 mg of rtPA, shows complete recanalization of all major arterial branches with only residual lack of opacification of a right frontal M4 branch.
It was the opinion of the interventional neuroradiologist and the referring neurologist that intraarterial thrombolysis would carry a significant risk of cerebral hemorrhage. However, we decided to proceed with intraarterial thrombolysis because of the relatively short duration of time since the onset of the stroke, the age and otherwise good health of the patient, and the extremely poor natural history of the disorder if left untreated. A baseline activated clotting time was obtained, and the patient was anticoagulated with 2000 U of heparin IV administered as a bolus infusion. Serial activated clotting times were obtained at 20-minute intervals, and additional heparin was administered as bolus infusions as necessary to maintain the activated clotting time in the 180- to 220-second range. An rtPA infusion consisting of 20 mg of rtPA in normal saline for a total volume of 50 mL was prepared. Intraarterial thrombolysis was started 2 hours 50 minutes after symptom onset. The microcatheter was withdrawn to the expected location of the right M1 bifurcation. Three milligrams of rtPA was slowly hand-injected through the microcatheter while the microcatheter was slowly withdrawn into the supraclinoid ICA during 5 minutes. The microcatheter was then advanced into the right A1 segment, and 1 mg of rtPA was injected through the microcatheter while the microcatheter was withdrawn into the supraclinoid ICA over 2 minutes. A digital subtraction angiography series obtained after hand injection of the microcatheter showed recanalization of the supraclinoid right ICA and the right A1 segment and persistent occlusion of the right M1 segment. The Excel 14 microcatheter was exchanged for a Micro-softstream microcatheter (Target Therapeutics) that had multiple side holes over the distal 3-cm segment of the catheter. The microcatheter was advanced over the microguidewire into the anterior and superior M2 branch so that the side holes traversed the M1 segment and the proximal M2 branch. With the catheter in this position, 13 mg of rtPA was infused at a rate of 10 mg/h.
Periodic digital subtraction series were obtained in the right ICA, and after infusion of the 13 mg of rtPA, recanalization of the right M1, the right anterior temporal artery, and the anterior and superior right M2 branch was achieved. The catheter position was changed so that it traversed the proximal occluded posterior and inferior right M2 branch. Five milligrams of rtPA was infused at 10 mg/h. A digital subtraction angiography series of the right carotid artery obtained after infusion of the 5 mg of rtPA showed recanalization of the M2 branch but persistent occlusion of an M3 branch supplying the posterior right frontal cortex. The catheter was advanced into the right M3 branch, and an additional 4 mg of rtPA was infused at a rate of 10 mg/h. A follow-up digital subtraction angiography series of the right carotid artery showed partial recanalization of the M3 branch but persistent occlusive thrombus in a distal M4 branch. However, it appeared that there were adequate pial collaterals to the distal cortical branches (Fig 1B). Considering the large amount of intraarterial rtPA already infused and the length of the procedure, the decision was made to terminate the procedure at that time because of the attendant high risk of cerebral hemorrhage if additional rtPA were to be infused. Intraarterial thrombolysis was terminated after 26 mg of rtPA had been infused and 5 hours 20 minutes after stroke onset. The catheters and sheath were removed from the right femoral artery, and hemostasis was obtained with a vascular closure device. The heparinization was allowed to reverse spontaneously. The patient was left intubated and was transported for a follow-up CT examination. CT was negative for hemorrhage or evolving areas of infarction. The patient was then transferred to the neurology intensive care unit and extubated electively.
Postprocedural Clinical Course
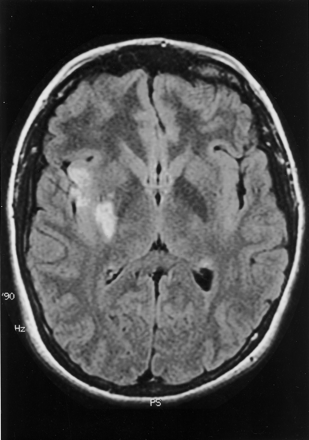
When the patient awoke from general anesthesia, only 1 hour after discontinuation of rtPA infusion and 6 hours 30 minutes after the onset of her symptoms, she had no deficits revealed by neurologic examination (National Institutes of Health Stroke Scale score of 0). IV administered heparin was restarted 24 hours after the angiographic procedure. MR images of the brain obtained 4 days later showed small areas of abnormal diffusion on diffusion-weighted images, with corresponding hyperintense signal on fluid-attenuated inversion recovery images, scattered in the right MCA distribution (Fig 2). Transesophageal ECG revealed the presence of a 5-mm patent foramen ovale with right-to-left shunt at rest that was markedly exacerbated during Valsalva maneuver. In addition, a redundancy of the adjacent atrial septum was consistent with an atrial septal aneurysm. A venous duplex of the lower extremities and a comprehensive hypercoagulability workup were unrevealing. Surgical closure of the patent foramen ovale was successfully performed during the hospital stay. Two weeks after experiencing a potentially devastating stroke, the patient was discharged, free of any neurologic deficits.
Fluid-attenuated inversion recovery (128/6000 [TR/TE]) MR image of the brain shows small and scattered areas of hyperintensity in the right MCA territory as the only residual sequelae 4 days after the event.
Discussion
Acute carotid T occlusion has been consistently associated with high rates of brain swelling, frequently followed by herniation and death (1, 2). It renders most of the ipsilateral carotid intracranial vascular territory ischemic, and collateral arterial supply via pial vessels is often inadequate. Furthermore, the medial and lateral lenticulostriate arteries that provide blood supply to the basal ganglia, which originate from the A1 and M1 segments, respectively, are end arteries with no source of collateral supply. Intraarterial thrombolysis delivered within 6 hours of stroke onset has proved successful in some patients with MCA occlusions (4), but this success has mostly not been replicated in cases of carotid T occlusion (5–8).
Kucinski et al (5) analyzed the predictive value of early CT and arteriographic features for fatal brain swelling in patients with acute stroke treated mostly with intraarterial thrombolysis. The study found that the presence of carotid T occlusion confirmed by angiography was the strongest predictor of mortality in their series (fatal swelling occurred in nine of 19 patients with carotid T occlusion). Patients with carotid T occlusion had a higher incidence of hemorrhage and very poor recanalization rates after thrombolysis. The authors concluded that fibrinolysis was not useful and was possibly even unsafe in cases of carotid T occlusion.
To our knowledge, full recovery after carotid T occlusion has never been specifically documented in the literature. Jansen et al (2) presented a series of 32 patients with carotid T occlusion, half of whom underwent intraarterial thrombolysis. Good outcomes (modified Rankin Scale scores of 0–3) were observed in only five (16%) of those patients, whereas almost one third had intracranial hemorrhage. In this study, thrombolytics usually failed to achieve recanalization and did not improve the prognosis for these patients. Gonner et al (8) reported good outcomes (also defined as modified Rankin Scale scores of 0–3) in three (33%) of nine patients with carotid T occlusion treated with intraarterial urokinase, although recovery did not correlate with degree of recanalization. The proposed explanation for the dismal outcome after carotid T occlusion in part relates to the size and composition of the clot. It has been postulated that large, cohesive, and rigid emboli are more prone to occlude the carotid bifurcation as the first major narrowing in the high-flow anterior circulation. Meanwhile, smaller, more flexible emboli are able to reach more distal branches of the arterial tree with or without previous fragmentation. The larger emboli are probably composed of aged thrombotic material that could be intrinsically less responsive to fibrinolytic agents (6). For our patient, the origin of the clot could not be documented, but the redundant atrial septum and the pelvic venous system were considered possible sources.
Our patient presented early after symptom onset with a severe neurologic deficit (National Institutes of Health Stroke Scale score of 23) and a hyperattenuated MCA sign revealed by CT. The presence a hyperattenuated MCA sign for patients with National Institutes of Health Stroke Scale scores greater than 10 has been associated with poor response to IV thrombolysis (9), prompting us to choose the intraarterial route for rtPA administration. Swift management allowed us to start the infusion less than 3 hours after stroke onset. This may explain the excellent outcome in this case, because the benefit of thrombolytics is maximized by earlier administration (10).
No clear consensus is presented in the literature regarding the appropriate total dose or rate of administration for intraarterial thrombolytics. This is understandable because variables such as time since onset of ictus, clinical neurologic status, site of occlusion, amount of thrombus to be lysed, and rapidity of lysis of the thrombus all play a role in determining the appropriate dose and rate of administration of intraarterial thrombolytics on a case-by-case basis. Some consensus exists regarding the appropriate dose for concomitant IV administered heparin arising from data reported in the PROACT I trial (11). An increased incidence of intracerebral hemorrhage was shown to occur in patients who received “high-dose” heparin (100 IU/kg bolus and then 1000 IU/h constant infusion for 4 hours) versus those patients who received “low-dose” heparin (2000-IU bolus and then 500 IU/h infusion). It has been our policy to administer a low dose of heparin as an initial bolus with subsequent frequent activated clotting time monitoring so that additional heparin can be administered as small boluses to maintain an activated clotting time in the 180- to 220-second range.
Intraarterial thrombolysis offers several advantages over the IV route by allowing direct infusion of the thrombolytic agent into the thrombus, a longer time window of administration, and the potential for combination with mechanical disruption or even angioplasty. Rates of recanalization, shown to correlate with better clinical outcome, are higher despite use of lower total doses of thrombolytics (our patient would have received 54 mg as opposed to 26 mg of rtPA had it been IV administered) (8). However, arterial catheterization may delay the delivery of the thrombolytic agent and may be associated with higher rates of intracerebral hemorrhage. In addition, it requires a degree of expertise that is usually available only in large tertiary centers. The use of low-dose IV rtPA as a bridge before intraarterial infusion has been proved feasible and safe (12) and is an option for smaller centers before transfer. This combined approach could be particularly valuable for patients with more severe strokes (National Institutes of Health Stroke Scale score, >10 or 15) (12).
Conclusion
We report the case of a young woman with severe neurologic deficits secondary to angiographically proved carotid T occlusion who recovered fully after the intraarterial administration of rtPA. Although this result could be regarded as exceptional, it suggests that early intraarterial thrombolysis may be effective in patients with carotid T occlusions and should be considered for appropriate candidates.
References
- Received March 25, 2002.
- Accepted after revision May 6, 2002.
- Copyright © American Society of Neuroradiology