Abstract
Summary: Numerous functional neuroimaging techniques have progressively been added to the presurgical evaluation of refractory partial epilepsies. These investigations can help confirm the origin of seizure onset previously suggested by MR imaging and electro-clinical data, provide independent prognostic information, and provide critical diagnostic value when MR imaging results are strictly normal or show multifocal abnormalities. Of the various functional neuroimaging modalities, [11C]methionine positron emission tomography for methionine uptake into seizure foci is still in the preliminary stages of investigation. A single case of medically intractable epilepsy with focal cortical dysplasia documented by [11C]methionine positron emission tomography and possible hypotheses to explain the methionine uptake into the seizure focus are described below.
An increasing number of functional neuroimaging techniques have progressively been added to the armamentarium of presurgical localization of the epileptic foci in patients with medically intractable epilepsies. Of the various functional neuroimaging modalities, [11C]methionine positron emission tomography for methionine uptake into seizure foci is still in the preliminary stages of investigation (1, 2). [11C]Methionine positron emission tomography has been reported to be one of the most useful tracers for the detection and management of brain tumors. Although the discovery of a high methionine uptake also in non-neoplastic lesions of the brain has been fortuitous (3–5), it nevertheless demands further studies on mechanisms of methionine uptake in regions of seizure foci. We report a single case of medically intractable epilepsy with focal cortical dysplasia documented by a battery of tests, including [11C]methionine positron emission tomography.
Case Report
A 34-year-old woman presented with medically intractable epilepsy of complex partial and left visual seizures occurring for nearly 32 years, with a frequency of from seven to 10 seizures per day to none for a few days. The longest seizure-free interval recorded was 1 week, and the patient continued to experience an average of one to two seizures per day. Except for mild to moderate impairment of general intellectual ability and a congenital left homonymous hemianopsia, no other focal neurologic deficits were observed.
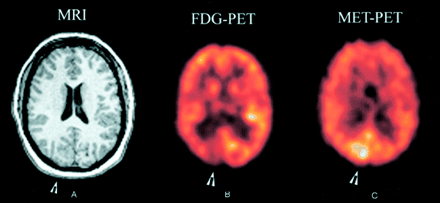
Contrast-enhanced MR imaging of the brain revealed abnormal thickening within the right occipital lobe, with extension of this process into the posterior aspect of the right temporal lobe. A neuronal migration disorder, such as polymicrogyria/pachygyria, was considered with a differential of a low grade glioma (Fig 1A).
Images from the case of a 34-year-old woman who presented with medically intractable epilepsy of complex partial and left visual seizures.
A, Contrast-enhanced MR image (MRI) of the brain shows thickening within the right occipital lobe and in the posterior aspect of the right temporal lobe, suggestive of polymicrogyria/pachygyria.
B, [18F]Fluorodeoxyglucose positron emission tomographic scan (FDG-PET) of the brain shows focal area of decreased glucose uptake in the right occipital lobe.
C, [11C]Methionine positron emission tomographic scan (MET-PET) of the brain shows enhanced methionine uptake in the right occipital lobe.
A 22-hour video-EEG monitoring study recorded eight clinical and electrographic seizures. Each of these seizures seemed to have an onset in the right posterior temporal occipital region. The clinical characteristics were also consistent with complex partial seizures arising in the right occipital region. Interictal abnormalities consisted of depression of the occipital rhythm on the right.
[11C]Methionine and [18F]fluorodeoxyglucose positron emission tomographic scans were obtained after administering 20 mCi and 10 mCi of [11C]methionine and [18F]fluorodeoxyglucose, respectively. Both studies were performed interictally, with no seizures having occurred for nearly 24 hr before scanning. No clinical or electrophysiological seizures occurred during the studies. A focal area of decreased glucose uptake was seen in the right occipital lobe, especially in the visual cortex (Fig 1B). This region also showed enhanced methionine uptake (Fig 1C). The pattern of uptake resembled that seen with low grade gliomas. The bilateral temporal lobe hypometabolism was greater on the left side. There was no concomitant methionine uptake in these areas. EEG performed during positron emission tomography revealed spikes occurring randomly and in rhythmic runs, frequently observed in the right occipital-posterior temporal regions.
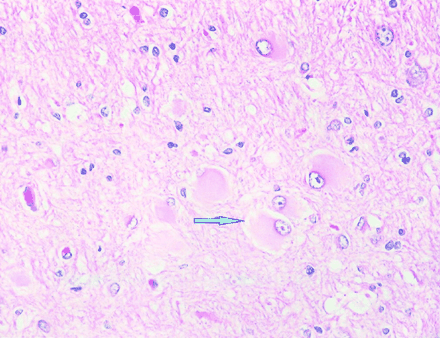
Subdural grid electrodes further circumscribed the seizure focus to predominantly the right mesial occipital cortex. The patient underwent subsequent right mesial occipital lobe resection. The excised tissue revealed typical findings of cortical dysplasia. In many of the coronal sections through the specimen, whole gyri were involved in a malformative process. The white matter in many of the sections contained numerous balloon type cells (Fig 2). Features of microdysgenesis were discernible in some areas.
Balloon cell changes (arrow). Balloon cells have eccentric nuclei and abundant opalescent eosinophilic cytomplasm (hematoxylin and eosin; original magnification, ×400).
Postoperatively, the patient continued to experience milder complex partial seizures, mostly nocturnal and at a decreased frequency of 1 to 2 per week. She underwent further electrophysiological studies, including a 94-hr video-EEG monitoring study with sphenoidal electrodes. This study revealed a more anterior focus on the right side, suggesting involvement of the right mesial temporal region. The patient subsequently underwent right mesial temporal cortex resection and became seizure-free after receiving antiepileptic drugs for 6 months postoperatively.
Discussion
The presurgical evaluation of drug-resistant partial epilepsies primarily relies on two major investigations, including a long-term video-EEG monitoring study, which aims at recording the patient’s typical seizures, and specifically designed high quality MR imaging. The latter reveals abnormalities within the epileptogenic lobe in the majority of cases, which might not necessarily match the epileptogenic zone. Numerous functional neuroimaging techniques have progressively been added to the presurgical evaluation of refractory partial epilepsies, such as the study of cerebral glucose metabolism, benzodiazepine receptor availability and methionine incorporation using positron emission tomography, the evaluation of ictal cerebral blood flow changes using single photon emission CT, the measurement of N-acetyl-aspartate concentration with MR spectroscopy, and the mapping of eloquent areas using functional MR imaging. These investigations can help confirm the origin of seizure onset previously suggested by MR imaging and electro-clinical data and provide independent prognostic information regarding the chance of successful surgical treatment. Moreover, functional neuroimaging data can have critical diagnostic value when MR imaging is strictly normal or shows multifocal abnormalities.
Methionine is well known to be one of the most effective tracers for evaluating brain tumors. [11C]Methionine accumulation was initially thought to depend on protein synthesis. However, other metabolic processes, including transmethylation processes with methionine as a methyl donor and transmembrane amino acid transport, are now thought to take part in methionine accumulation (6). Recent reports documenting high [11C]methionine uptake in non-neoplastic tissues, such as brain abscess, intracerebral hematomas, cerebral ischemia, and brain radiation necrosis (1, 3–5), have suggested that high methionine uptake cannot always rule out the presence of a non-neoplastic lesion.
Focal cortical dysplasia (cortical dysplasia with neuronal cytomegaly), as described by Taylor et al (6) as being present in specimens from resections for the treatment of medically intractable partial epilepsy, is a sporadic disorder that has characteristic histologic features. The lesion may be seen macroscopically and may be represented by wider than normal gyri or an area of partial lissencephaly. The histologic features include disturbed cortical lamination and large abnormal neurons, with prominent processes present throughout the cortex and often extending into the adjacent white matter. These are usually accompanied by large balloon cells with glassy eosinophilic cytoplasm and pleomorphic eccentric nuclei that are often restricted to the deeper layers of the cortex and adjacent white matter. The nature of these cells is ambiguous, as these may express both glial and neuronal markers (7, 8). The large neurons are strongly immunoreactive for high and medium molecular weight neurofilament epitopes (9).
Sasaki et al (2) compared [11C]methionine positron emission tomography with [18F]fluorodeoxyglucose positron emission tomography and technetium-99m-electrochemical detection single photon emission CT in four patients with pathologically proven cortical dysplasias. On [11C]methionine positron emission tomographic scans, all four lesions were visually recognized to have high [11C]methionine uptake areas. The same authors also reported a case of radiation necrosis with epileptic activity showing a high methionine uptake (1). Although this form of cortical dysplasia is more of a proliferative disorder that often is confused with dysembryoplastic neuroepiethelial tumor, definite pathologic features existed in the present study to clearly distinguish between the two. Dysembryoplastic neuroepiethelial tumors are unique and benign congenital tumors, occurring frequently in children and adolescents. Kaplan et al (10) studied five patients with confirmed dysembryoplastic neuroepiethelial tumors using [18F]fluorodeoxyglucose and [11C]methionine positron emission tomography in an attempt to categorize these tumors metabolically. Results of the study concluded that they are inactive tumors with no significant glucose or metabolic activity.
The exact mechanisms for the increased methionine uptake in focal cortical dysplasia remain unclear. Postulated hypotheses include the following: 1) subacute gliotic reaction surrounding seizure focus may be responsible for increased uptake of [11C]methionine; 2) epileptic activity can also be a possible reason for the high methionine uptake (2), and seizure activity may cause alteration in amino acid transport mechanisms, inflammatory changes, or even hyperperfusion; 3) seizure activity is related to an increase in sodium ion influx into the cell as a consequence of membrane depolarization and increased NaK ATPase activity, and it is hypothesized that methionine is concomitantly carried in with the Na+ ion influx; 4) disruption of blood brain barrier may result in leakage of [11C]methionine to the extracellular space, enabling increased uptake in the cells; 5) alterations in amino acid uptake in the brain in relation to aging have been described, and age-related decline occurs in the uptake of [11C]methionine in the brain (11–13), with the high [11C]methionine uptake observed in areas of focal cortical dysplasia possibly indicating that these areas represent developmentally abnormal immature tissues.
Although these hypothetical mechanisms may account for the high uptake in the mesial occipital region, the areas of temporal hypometabolism seen on [18F]fluorodeoxyglucose-positron emission tomographic scans and not on methionine scans remain unexplained. This area also needed to be resected before optimal seizure control could be obtained. This would indicate either that at the time of the initial study, the predominantly active seizure focus was the mesial occipital lobe, which therefore also showed methionine uptake, or that the extent of immature brain tissue, which presumably governed the uptake of the amino acid in the mesial temporal lobe areas, was not adequate. Unfortunately, a repeat methionine scan was not obtained for our patient before the resection of the mesial temporal lobe. Although there is no evidence to support these mechanisms, it is interesting to postulate that an active seizure focus will be evident as a high methionine uptake area in cases of focal cortical dysplasia. The mechanisms of [11C]methionine uptake into seizure foci need to be further delineated.
Footnotes
This effort was sponsored by the Air Force Research Laboratory (AFRL/HEOP), Air Force Material Command, United States Air Force, under cooperative agreement F33615–98-2–6002.
References
- Received May 30, 2001.
- Accepted after revision November 26, 2001.
- Copyright © American Society of Neuroradiology