Abstract
BACKGROUND AND PURPOSE: Subcortical low-intensity lesion on T2-weighted images is an uncommon manifestation of ischemia, multiple sclerosis, and Sturge-Weber disease. This study was performed to determine whether subcortical low signal intensity is an MR feature of meningitis, viral encephalitis, or leptomeningeal metastasis and to investigate a cause of subcortical low intensity.
METHODS: We retrospectively reviewed MR images of 117 patients with meningitis, encephalitis (viral or unknown), or leptomeningeal metastasis for the presence of subcortical low intensity, meningeal enhancement, signal intensity change of cortex, and change in subcortical low intensity on follow-up images. Diffusion-weighted (DW) images and apparent diffusion coefficient (ADC) maps were obtained in 55 patients. Subcortical low-intensity lesions were also quantitatively analyzed on T2-weighted, fluid-attenuated inversion recovery (FLAIR), and DW images.
RESULTS: Subcortical low intensity was found in nine (23.7%) of 38 patients with encephalitis (viral, 31; unknown origin, 7), five (24%) of 21 with leptomeningeal metastasis, and five (9%) of 58 with meningitis. Leptomeningeal enhancement was observed in 100% and cortical hyperintensity in 14 (74%) of 19 patients with subcortical low intensity. Leptomeningeal enhancement was seen in 46 (47%) and cortical hyperintensity in 33 (34%) of 98 patients without subcortical low intensity. Subcortical low intensity disappeared or decreased in extent on follow-up MR images in all seven patients who underwent follow-up. ADC of subcortical low-intensity lesions was lower than that of the contralateral area and decreased by 9.3 ± 11.4%.
CONCLUSION: Subcortical low intensity was uncommonly found in meningitis, viral encephalitis, and leptomeningeal metastasis. It is a nonspecific MR sign of various meningeal and cortical diseases. Although the cause of subcortical low intensity remains uncertain, free radical formation may play a role as a causative factor.
Subcortical low-intensity lesions on T2-weighted images have been reported as an uncommon MR finding in patients with Sturge-Weber syndrome (1), early cortical ischemia and infarction (2, 3), moyamoya disease (4, 5), severe ischemic-anoxic insults (6), and multiple sclerosis (7). Ida et al (3) suggested that subcortical low intensity was an important diagnostic sign of early cortical ischemia. Although nonheme iron accumulation by the interruption of axonal transport and free radical formation were suggested as causes (2–7), the pathogenesis of subcortical low intensity is still uncertain.
The purpose of this study was to determine whether subcortical low intensity is an MR feature of meningitis, viral encephalitis, and leptomeningeal metastasis and to investigate the pathogenesis of subcortical low intensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images.
Methods
We retrospectively reviewed MR images of 117 consecutive patients in whom the final diagnosis was encephalitis, meningitis, or leptomeningeal metastasis during a period of two years (January 1999 to December 2000). There were 42 female and 75 male patients with a mean age of 41 years (range, 1–68 years). Final clinical diagnoses of the patients included in the study were meningitis in 58 patients, encephalitis in 38, and leptomeningeal metastasis in 21. The final diagnosis of encephalitis or meningitis was made on the basis of clinical features, laboratory findings including CSF studies, and histopathologic examination. The diagnosis of leptomeningeal metastasis was made when malignant cells were present at cytologic examination of CSF.
MR imaging was performed with two 1.5-T MR imaging systems (Magnetom Vision; Siemens, Erlangen, Germany and Signa Horizon; GE Medical Systems, Milwaukee, WI). In all patients, spin-echo T1-weighted, fast spin-echo T2-weighted, and fast FLAIR images were obtained. Enhanced T1-weighted images with gadopentetate dimeglumine (Magnevist; Schering, Berling, Germany) at a dose of 0.1 mmol/kg were also obtained in all patients. Echo-planar diffusion-weighted (DW) images and apparent diffusion coefficient (ADC) maps were obtained in 55 patients.
The MR imaging parameters were as follows: 450–466/12/2 (TR/TE/NEX) for spin-echo T1-weighted images, 3666–4000/96–104/1–2/7 (TR/TEeff/NEX/echo train) for fast spin-echo T2-weighted images, and 9000–10000/110–123/2200–2610/1/7 (TR/TEeff/TI/NEX/echo train) for fast FLAIR images. Other parameters were a section thickness of 5–6 mm with a gap of 2.0–1.2 mm, a field of view of 210 mm, and a matrix of 256 × 192. The isotropic DW images were obtained by using single-shot echo-planar spin-echo sequences with parameters of 5400–6500/103–98.9/1 (TR/TE/NEX), a high b value (b = 1000 s/mm2), a 5–6-mm section thickness with a 1.2–2.0-mm gap, a 250- or 280-mm field of view, and a 128 × 128 matrix.
Two radiologists (J.H.L., D.G.N.) evaluated the signal intensity of subcortical white matter, meningeal enhancement, and signal intensity of cortex, in consensus. For determining the presence of a subcortical low-intensity lesion, subcortical discrete nodular or linear low-signal-intensity lesions, indicating old hemorrhage or calcification, were excluded in this study. The signal intensity change of the subcortical low-intensity lesion was also assessed in patients who underwent follow-up MR examinations. MR findings of leptomeningeal enhancement and cortical hyperintensity were compared between patients with and those without subcortical low-intensity lesions.
Quantitative analysis of subcortical low-intensity lesions was performed on T2-weighted, fast FLAIR, and DW images. The signal intensity was measured with three paired regions of interest. A 3–4-mm region of interest was carefully placed in an area of subcortical white matter with a reduced signal intensity and in a matched area of contralateral normal-appearing subcortical white matter. When the subcortical low-intensity lesions were bilateral, another area of normal-appearing white matter was selected as the reference.
The ADC was measured in all patients in whom DW images were obtained and was calculated from the subcortical low-intensity lesions and the contralateral normal white matter according to the following equation: ADC = ln(S0/S1)/(b1 − b0), where S0 and S1 are the signal intensities on the two DW images with b0 = 0 and b1 = 1000 s/mm2, respectively. In the patients without subcortical low intensity, ADC of the lesion was calculated in the subcortical white matter near leptomeningeal enhancement or cortical hyperintensity. When there was no demonstrable leptomeningeal enhancement or cortical lesion, ADC of the lesion was calculated in the subcortical white matter of the right frontoparietal lobe. The signal intensities and ADC values of the subcortical low-intensity lesion and contralateral control area were compared with use of a Student t test. A P value of less than .05 was considered to indicate a statistically significant difference.
In one patient, a surgical biopsy of brain was performed for the final diagnosis 4 days after initial MR examination. In this patient, the resected brain specimens were stained with hematoxylin-eosin, and histochemical studies of Luxol fast blue and Perls iron stain were performed on the surgical specimen. Immunohistochemical studies of CD3, CD20, CD68, glial fibrilllary acid protein, and neurofilaments were also performed for pathologic interpretation. The histologic features of subcortical white matter and cortex of the specimens were evaluated by a pathologist (Y.-L.S.).
Results
Table 1 shows the distribution of subcortical low-intensity lesions among the 117 patients with meningitis, encephalitis, or leptomeningeal metastasis. Among 31 patients with viral encephalitis, subcortical low intensity was found in two (12%) of 17 patients with herpes encephalitis and in seven (50%) of 14 patients with other viral encephalitis. Among patients with meningitis, subcortical low intensity was found in the patients with various kinds of meningitis except viral meningitis. In five patients with leptomeningeal metastasis in whom low intensity of subcortical white matter was found, the cytologic examinations of CSF revealed adenocarcinomas (lung cancer in four and gastric cancer in one) in all patients. In 19 patients with subcortical low-intensity lesions, initial MR images were obtained at the acute stage (<7 days after symptom onset) in seven patients and at the subacute stage (7–30 days) in 10 patients. In two patients, subcortical low-intensity lesions were found on follow-up MR images that were obtained at the chronic stage (50 and 60 days after initial symptom onset and medical therapy).
TABLE 1: Distribution of subcortical low-intensity lesions in 117patients with encephalitis, meningitis, or leptomeningeal metastasis
In the visual assessment of the images in the 19 patients with subcortical low intensity, the fast spin-echo T2-weighted and fast FLAIR images showed focal or patchy ill-defined low signal intensity in the subcortical white matter (Figs 1–4). The T1-weighted images revealed no visible signal intensity change in the subcortical white matter in all patients. On DW images, the subcortical low-intensity lesions were hypointense in six (43%) of 14 patients (Fig 1) and were isointense in the remaining eight patients (87%).
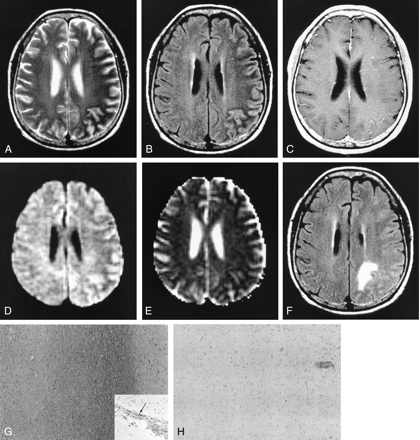
Images in a 60-year-old woman with viral encephalitis. MR images in A–E were obtained at the subacute stage (13 days after symptom onset).
A, Axial T2-weighted image shows asymmetric confluent low intensity in the left frontoparietal lobe.
B, FLAIR image also shows subcortical low intensity with diffuse cortical high intensity in the affected hemisphere.
C, Axial contrast-enhanced T1-weighted image shows mild leptomeningeal enhancement in the left hemisphere.
D, DW image shows mild hypointensity in the subcortical white matter in the left hemisphere.
E, ADC map also shows mild hypointensity of the subcortical low-intensity lesions. Mean ADC value of the subcortical lesions was 0.65. Hypointensity of the subcortical low-intensity lesion on the DW image in spite of low ADC may be explained by the low-intensity T2 shine-through effect.
F, Follow-up FLAIR image obtained 14 days after the initial MR examination shows that the subcortical low intensity is no longer demonstrated but has changed to intermediate to slightly high signal intensity. Localized edema is seen at the biopsy site in the left parietal lobe. Leptomeningeal enhancement was not demonstrated on the follow-up contrast-enhanced T1-weighted image (not shown).
G, Photomicrograph of the surgical specimen obtained 4 days after the initial MR examination shows no structural abnormality in the subcortical white matter except for focal myelin pallor (Luxol fast blue stain, original magnification ×40). Inset shows perivascular mononuclear cell infiltration (arrow) in the white matter (immunohistochemical stain, original magnification ×200). The cells are reactive for CD3, a marker for T lymphocyte.
H, Photomicrograph from histochemical study of iron stain reveals no evidence of accumulated iron in the cortex and subcortical white matter (Perls stain, original magnification ×40).
The distribution of MR findings of cortical hyperintensity and leptomeningeal enhancement in each patient group are summarized in Table 2. In the patient group with subcortical low-intensity lesions, a variable degree of leptomeningeal enhancement was found near subcortical low-intensity areas on enhanced T1-weighted images in all patients, and it was more prominent in leptomeningitis and leptomeningeal metastasis (Figs 3 and 4). Pachymeningitis was found in two patients with pyogenic meningitis and meningitis of unknown cause, as well as subdural empyema and subdural fluid, respectively (Fig 2). In the group with subcortical low intensity, although both leptomeningeal enhancement and cortical hyerintensity were found in most patients (89–100%) with meningitis or encephalitis, cortical hyperintensity near subcortical low-intensity lesions was found in only one (20%) of five patients with leptomeningeal metastasis. In the patient group without subcortical low-intensity lesions, leptomeningeal enhancement and cortical hyperintensity were less frequently found than in the patients with these lesions. In patients without these lesions, MR findings of cortical hyperintensity were found in many patients but associated leptomeningeal enhancement was relatively uncommon in encephalitis.
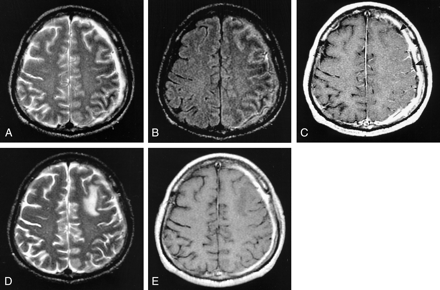
Images in a 50-year-old man with empyema and chronic meningitis. MR images in A–C were obtained at the subacute stage (19 days after symptom onset).
A, Axial T2-weighted image shows low intensity in subcortical white matter in the left frontoparietal lobe.
B, Axial FLAIR image also shows low intensity in the left frontoparietal lobe with high intensity of cortex and sulcus.
C, Axial contrast-enhanced T1-weighted image shows prominent enhancement of thickened pachymeninx and leptomeningeal enhancement in the affected hemisphere. Small amount of subdural fluid (arrowheads) is also seen in the left hemisphere.
D, Follow-up T2-weighted image obtained 19 days after initial MR examination shows that the subcortical low intensity is markedly decreased. Focal edema is noted in the left frontal lobe; this might be the result of parenchymal injury developed during meningeal biopsy. Histologic examination revealed nonspecific chronic meningeal inflammation (not shown).
E, On follow-up contrast-enhanced T1-weighted image, no leptomeningeal enhancement is seen.
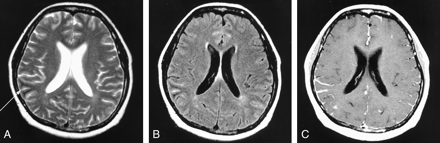
Images in a 27-year-old man with cryptococcal meningitis.
A–C, MR images obtained at the chronic stage (60 days after initial symptom onset).
A, T2-weighted image shows subcortical low intensity (arrow) and adjacent cortical hyperintensity in the right occipital lobe.
B, Axial FLAIR image also shows low intensity (arrow) in the same area.
C, Axial contrast-enhanced T1-weighted image shows prominent leptomeningeal enhancement adjacent to the subcortical low-intensity lesion.
D, On follow-up FLAIR image, obtained 30 days after initial MR examination, subcortical low intensity is no longer demonstrated.
E, On follow-up contrast-enhanced T1-weighted image, leptomeningeal enhancement also is no longer demonstrated.
Images in a 49-year-old women with leptomeningeal carcinomatosis from adenocarcinoma of the lung. MR images in A–C were obtained at the acute stage (4 days after symptom onset).
A, Axial T2-weighted image shows localized subcortical low intensity (arrow) in the right parietal lobe.
B, Axial FLAIR image also demonstrates subcortical low intensity in the same area, with adjacent sulcal hyperintensity. Hyperintensity of cortex is not demonstrated on T2-weighted or FLAIR images.
C, Contrast-enhanced T1-weighted image demonstrates diffuse leptomeningeal enhancement in both hemispheres. Strong leptomeningeal enhancement adjacent to the subcortical low-intensity lesion is also seen in the right parietal lobe.
TABLE 2: Leptomeningeal enhancement and cortical hyperintensity on MR images
Follow-up MR images were obtained in seven (four with encephalitis and three with meningitis) of 19 patients, but not obtained in any patients with leptomeningeal metastasis. Follow-MR images were obtained 14, 19, 22, 30, 35, 38, and 85 days after MR images that showed subcortical low-intensity lesions. On follow-up MR images, the signal intensity of the subcortical low-intensity lesions increased in all seven patients, becoming nearly isointense in five patients and hyperintense in two patients. Cortical hyperintensity was found in six of the seven patients on initial MR images, and it persisted in two patients with viral encephalitis in whom the subcortical low intensity disappeared on follow-up MR images (Fig 1). Meanwhile, the leptomeningeal enhancement disappeared or markedly decreased on follow-up MR images in all patients (Figs 2 and 3).
In the quantitative analysis, the signal intensity in the subcortical low-intensity area was reduced relative to that of the corresponding contralateral white matter on the fast spin-echo T2-weighed and fast FLAIR images by 14% ± 6.7 and 15.1% ± 8.7, respectively (P < .00001 and P < .00001, respectively). There was no significant difference in the percentage of signal intensity decrease between T2-weighted and fast FLAIR images (P > .05). The mean ADC of subcortical low-intensity lesions was slightly lower than that of the contralateral hemisphere (P < .00001) (Table 3). In the patient group without subcortical low intensity, no significant difference was noted between the ADC of lesion and that of contralateral subcortical white matter. The significant decrease in ADC (<10%) (8) was found in seven (50%) of 14 patients with subcortical low intensity, but was not found in any patient without subcortical low intensity.
TABLE 3: ADC changes of subcortical low-intensity lesions in 55 patients
In one patient with viral encephalitis who underwent brain biopsy, the histologic examination revealed no obvious evidence of structural abnormality or cytotoxic edema in the subcortical white matter (Fig 1). There was focal myelin pallor in the subcortical white matter on a histochemical study with Luxol fast blue stain, and perivascular mononuclear cell infiltrations were positive on immunohistochemical studies with CD3, CD20, and CD68. There was no evidence of increased iron in the cortex or subcortical white matter on a histochemical study of Perls iron stain. The brain cortex showed neuronal loss, microglial cell proliferation with neuronophagia, and mononuclear cell infiltration; there was also mononuclear cell infiltration in the leptomeninges. An immunohistochemical study of glial fibrillary acid protein showed a positive stain in reactive astrocytes.
Discussion
Our results suggest that various intracranial diseases of infection and malignancy should be included in the differential diagnosis for a cortical or leptomeningeal lesion that shows subcortical low intensity on T2-weighted or fast FLAIR MR images.
The MR finding of subcortical low intensity was present in meningitis, viral encephalitis, and leptomeningeal metastasis in our study, in addition to the diseases previously reported (1–7). Among these diseases, subcortical low intensity was most commonly manifested in viral encephalitis, excluding herpes encephalitis. Subcortical low intensity was uncommon in leptomeningeal metastasis and herpes encephalitis and was rare in meningitis. Although subcortical low intensity was reported as an important diagnostic sign of early cortical ischemia (3), the results of our study indicate that the MR sign of subcortical low intensity can result from various abnormalities involving the cerebral cortex or meninges. Viral encephalitis should be included in the differential diagnosis of hyperintense cortical lesion with subcortical low intensity and should not be misinterpreted as acute or subacute cerebral ischemia. Although DW imaging is very sensitive and accurate for the diagnosis of early cortical ischemia, cortical hyperintensity may be ambiguous in the subacute stage of infarction, and cortical hyperintensity may be found in encephalitis (9).
In our study, subcortical low-intensity lesions were accompanied by prominent leptomeningeal enhancement in all patients and cortical hyperintensity in many patients. This possibly suggests that subcortical low intensity may be related to the meningeal or cortical abnormality that influences the biologic or metabolic state of the subcortical white matter.
Accumulation of nonheme iron and free radical formation have been proposed as the causative factors for subcortical low intensity in cerebral ischemia (2–6). Cross et al (2) suggested that profound low intensity in the deep gray matter and white matter on long TR/TE images in infarction were due to the deposition of nonheme iron possibly because of the interruption of the axonal iron projections, free radical–mediated direct tissue injury, and Wallerian degeneration. Ida et al (3) reported on nine cases with subcortical low intensity in early cortical ischemia. They hypothesized that the continuous production of a large number of free radicals during the acute and early subacute stages and iron deposition caused by the interruption of axonal transport could be responsible for lower signal intensity on T2-weighted images during the late subacute and chronic stages (3). However, the results of a recent animal study (10) do not support a hypothesis that disruption of the axonal transportation of iron may induce deposition of iron in the subcortical white matter. Palmer et al (10) reported that there was accumulation of iron only in the cortex, but iron accumulation was not obvious in the white matter in a rat after hypoxic-ischemic brain injury.
Although the mechanism of subcortical low intensity also seems to be unclear in encephalitis and meningeal diseases, the results of our study provide information for understanding subcortical low intensity in these diseases. First, MR features of subcortical low intensity were seen in meningeal diseases (meningitis and leptomeningeal metastasis) as well as cortical disease (encephalitis). This indicates that a common causative factor for subcortical low intensity may exist in various diseases involving the cerebral cortex or meninges. Second, our study showed that subcortical low intensity was transient in the acute or subacute stage in all patients who underwent subsequent follow-up MR imaging. Third, cortical hyperintensity persisted in some patients when the subcortical low intensity disappeared on follow-up MR images. Meanwhile, disappearance or decrease of subcortical low intensity was accompanied by the decrease of leptomeningeal enhancement at follow-up. Fourth, histologic examination in one patient revealed no obvious evidence of structural abnormality or accumulation of iron in the subcortical white matter. This histologic finding supports a hypothesis that subcortical low intensity is not caused by structural change of subcortical white matter or by a paramagnetic substance of accumulated iron.
As a common mechanism of subcortical low intensity in the diseases involving cortex and meninges, a paramagnetic substance as seen in free radicals can be suggested as causative because oxygen free radicals are implicated as important pathologic mediators in meningitis, encephalitis (11–19), subarachnoid hemorrhage (20–23), malignancy (24, 25), and trauma (26). It has also been reported (27–29) that the production of a large amount of free radicals can occur transiently over a certain period, which indicates that the transient presence of a subcortical low-intensity lesion may be explained by a transient increase in the amount of free radicals. The persistent cortical hyperintensity in patients in whom subcortical low intensity and leptomeningeal enhancement have disappeared on follow-up images suggests that the subcortical low intensity found in encephalitis or meningitis seems to be strongly related to the activity of disease involving the cortex or meninges rather than structural abnormality of cortex. These characteristics of subcortical low intensity will support a hypothesis that free radicals may play an important role in the production of subcortical low intensity in encephalitis or meningitis. We think that iron accumulation alone caused by axonal injury as reported previously (2–7) is insufficient for the explanation of the characteristics of subcortical low-intensity lesions described above because it may not occur in a short period and would not be reversible (3); in addition, it is a less likely explanation of subcortical low intensity in patients with no obvious cortical change on MR images.
The question arises as to the reason why subcortical low intensity is uncommonly observed in patients with encephalitis, meningitis, and leptomeningeal carcinomatosis. Oxygen free radicals are constantly produced at low concentrations as part of the normal metabolism and are scavenged by endogenous antioxidants such as superoxide dismutase and glutathione peroxidase (12, 14). Subcortical low intensity may be visualized only when there is a sufficient concentration of free radicals and ferric iron produced at a certain time in the disease process without sufficient free radical scavengers or iron chelators. The reason is uncertain why cortical low intensity is not found in spite of the presence of free radicals in the cortex as well as white matter. It may be partly explained by cortical edema caused by infection or malignancy, which will cause increase of the cortical signal intensity, and this may surpass or mask T2-shortening effect by free radicals.
The subcortical low-intensity lesions had intermediate or low signal intensity on DW images, and the measured ADCs were slightly lower than those of the normal white matter. The reason why the signal intensity of subcortical low-intensity lesion was not high on DW images in spite of low ADCs may be explained by the shine-though effect of subcortical low intensity on T2-weighted images. The reason for low ADC, however, is uncertain. On the basis of the histologic findings in one patient (Fig 1), we speculate that the low ADC seems not to be caused by structural change such as cytotoxic edema in the subcortical white matter. If a paramagnetic substance such as free radicals exists within subcortical low-intensity lesions, the ADC of these lesions may not be accurately determined owing to the influence of a paramagnetic substance (30). Further investigation is necessary for determining the cause of the low ADC.
A limitation of our study is that follow-up MR images were obtained only in a limited number of patients and were not obtained in patients with leptomeningeal metastasis. If subcortical low intensity is caused by a large amount of free radicals, the subcortical low-intensity lesion may indicate a potentially injured tissue because free radicals have a cytotoxic effect. Further study may be needed to determine the clinical significance of subcortical low intensity, and the factors causing it may be clarified by analyzing serial MR images in a larger cohort of patients.
Conclusion
Subcortical low-intensity lesion on long TR/TE images was an uncommon finding in various diseases including viral encephalitis, meningitis, and leptomeningeal metastasis. The MR feature of subcortical low intensity may be a nonspecific sign of various cortical and meningeal diseases. In viral encephalitis and meningitis, subcortical low intensity was commonly associated with leptomeningeal enhancement and cortical hyperintensity, and it seems a transient phenomenon at the acute or subacute stage. Although the cause of subcortical low intensity remains uncertain, free radicals may play a role as a causative factor.
References
- Received August 31, 2001.
- Accepted after revision January 2, 2002.
- Copyright © American Society of Neuroradiology